Calprotectin-A heterodimeric complex composed by S100A8/S100A9, a tool for inflammatory disease research
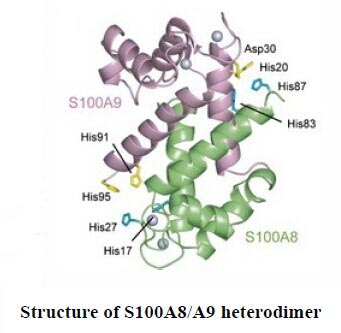 Calprotectin, usually known as S100A8/A9, is a heterodimeric complex composed by two components, S100A8 and S100A9. These two proteins are calcium- and zinc-binding proteins from the S100 protein family that are released from neutrophils under inflammatory conditions. It has been reported that amounts of S100A8/A9 in blood or extracellular body fluid are increased under many pathological conditions, for instance, rheumatoid arthritis[1], inflammatory bowel diseases[2], viral or microbial infections[3,4], tumors[5], and many inflammatory conditions[6]. Notably, abscess fluids contain more than 1mg/mL of the protein complex[7]. Therefore, the measurement of Calprotectin could be considered as an important tool for inflammatory disease research.
Calprotectin, usually known as S100A8/A9, is a heterodimeric complex composed by two components, S100A8 and S100A9. These two proteins are calcium- and zinc-binding proteins from the S100 protein family that are released from neutrophils under inflammatory conditions. It has been reported that amounts of S100A8/A9 in blood or extracellular body fluid are increased under many pathological conditions, for instance, rheumatoid arthritis[1], inflammatory bowel diseases[2], viral or microbial infections[3,4], tumors[5], and many inflammatory conditions[6]. Notably, abscess fluids contain more than 1mg/mL of the protein complex[7]. Therefore, the measurement of Calprotectin could be considered as an important tool for inflammatory disease research.
As Calprotectin is a hetero dimer constituted by two subunits, it has been a worldwide puzzle to establish the intact structure of the complex by biological technique. We Cloud-Clone Corp. have constructed recombinant proteins S100A8 and S100A9, respectively and connected the two proteins by a special linker. Multiple verification tests showed that we successfully obtained a complex (RPK504Hu01)which mimic the native structure of Calprotectin. This product has been purchased by a scientist at HUST and it has obtained the user's approval and praise.
Furthermore, specific monoclonal antibodies to Calprotectin were obtained by hybridoma technique and were utilized in ELISA Kit(SEK504Hu) for detection of human Calprotectin. The product lines about Calprotectin include: recombinant protein and Elisa Kit, please check out detailed information on For more detailed, you can visit www.cloud-clone.us
In the meanwhile, some other proteins, with high homology but not 100% homology in different species, are still in further research and development. For example, Transforming Growth Factor Beta 1, it shares 100% homology in mouse and rat, but this sequence shares 99% homology in human, pig, bovine, and sheep, and 98% in guinea pig (Picture 1). Moreover, this protein displays the same tertiary structure in different species, shown in picture 2.
Reference
1. Fagerhol MK, Munthe E, Berntzen HB, Olmez U. The leukocyte protein L1 in plasma and synovial fluid from patients with rheumatoid arthritis and osteoarthritis.Scand J Rheumatol. 1991;20(2):74–82.
2. Aadland E, Schjønsby H, Røseth AG, Fagerhol MK. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27(9):793–798.
3. Aukrust P, Fagerhol MK, Müller F, Frøland SS. Elevated serum calprotectin levels in HIV-infected patients: the calprotectin response during ZDV treatment is associated with clinical events. J Acquir Immune Defic Syndr. 1997;7(9):931–939.
4. Bakken JS, Dale I, Sander J, Fagerhol MK. Plasma levels of the leucocyte L1 protein in febrile conditions: relation to aetiology, number of leucocytes in blood, blood sedimentation reaction and C-reactive protein. Scand J Clin Lab Invest. 1984 ;44(4):357–362.
5. Koupilova K, Stulik J, Osterreicher J, et al. The analysis of S100A9 and S100A8 expression in matched sets of macroscopically normal colon mucosa and colorectal carcinoma: the S100A9 and S100A8 positive cells underlie and invade tumor mass.Electrophoresis. 1999;20(4–5):1047–1054.
6. Lyberg T, Johne B, Fagerhol MK, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50(3):113–123.
7. Golden BE, Clohessy PA. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol. 1995;42(5):551–556.
