Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy

On December 6, 2021, Li Tang, Institute of Materials Science and Engineering, École polytechnique fédérale de Lausanne (EPFL), and his team published an article titled “Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy” in Nature Biomedical Engineering, which revealed a mechanical immune checkpoint that could be targeted therapeutically to improve the effectiveness of cancer immunotherapies.

The protein [Active Perforin 1 (PRF1), APB317Mu01] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.

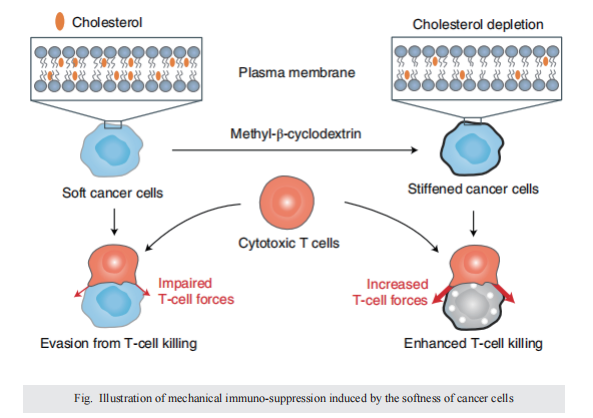
Malignant transformation and tumour progression are associated with cancer-cell softening. Yet how the biomechanics of cancer cells affects T-cell-mediated cytotoxicity and thus the outcomes of adoptive T-cell immunotherapies is unknown. Here we show that T-cell-mediated cancer-cell killing is hampered for cortically soft cancer cells, which have plasma membranes enriched in cholesterol, and that cancer-cell stiffening via cholesterol depletion augments T-cell cytotoxicity and enhances the efficacy of adoptive T-cell therapy against solid tumours in mice. We also show that the enhanced cytotoxicity against stiffened cancer cells is mediated by augmented T-cell forces arising from an increased accumulation of filamentous actin at the immunological synapse, and that cancer-cell stiffening has negligible influence on: T-cell-receptor signalling, production of cytolytic proteins such as granzyme B, secretion of interferon gamma and tumour necrosis factor alpha, and Fas-receptor–Fas-ligand interactions. Our findings reveal a mechanical immune checkpoint that could be targeted therapeutically to improve the effectiveness of cancer immunotherapies.