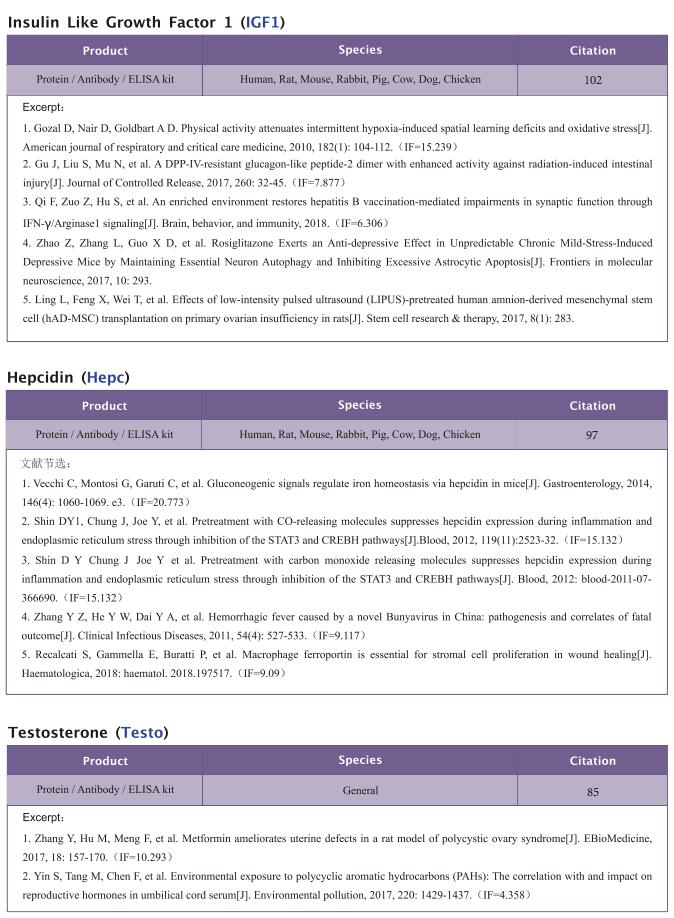
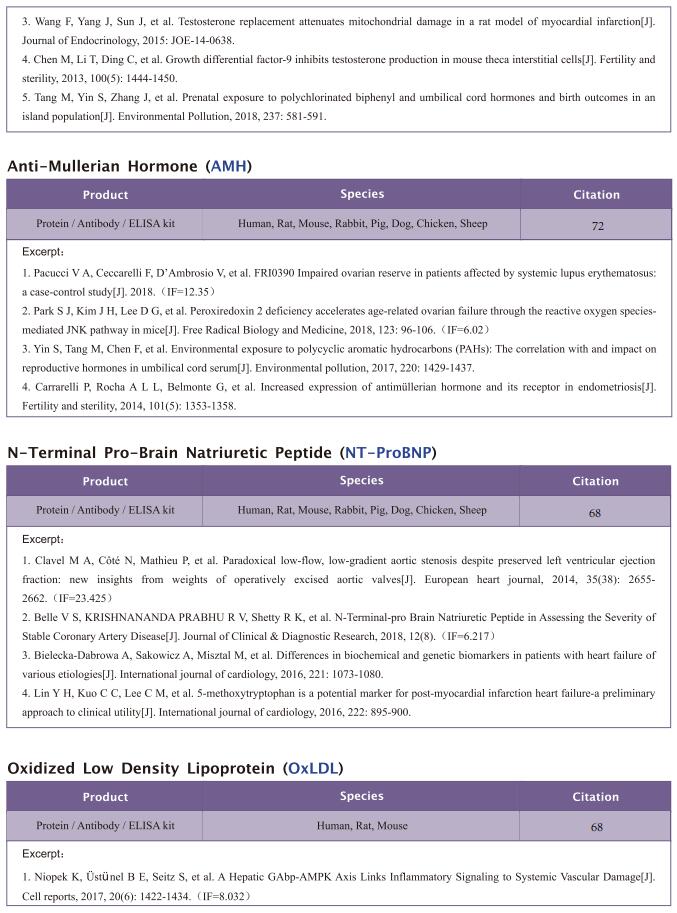
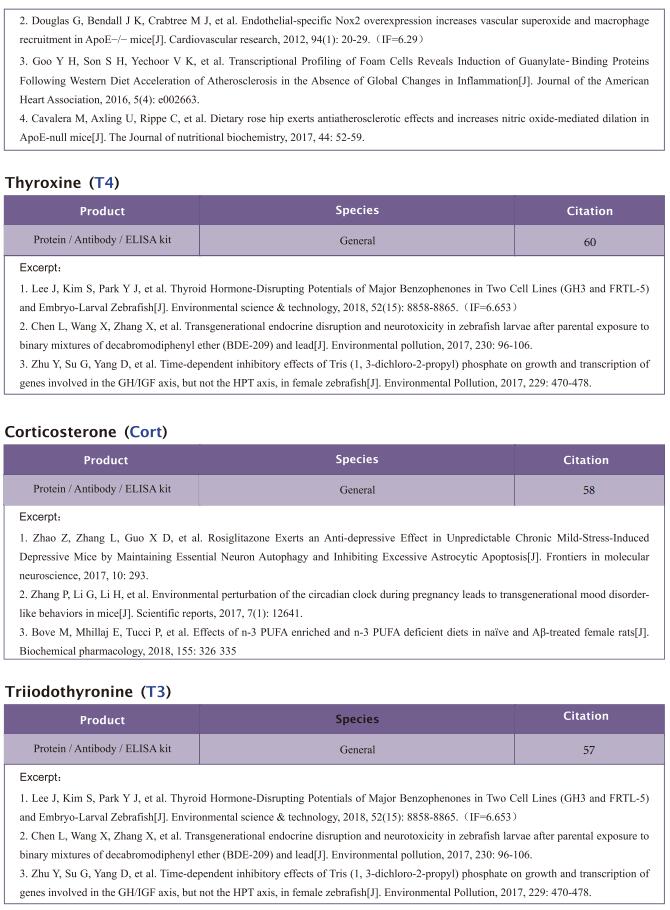
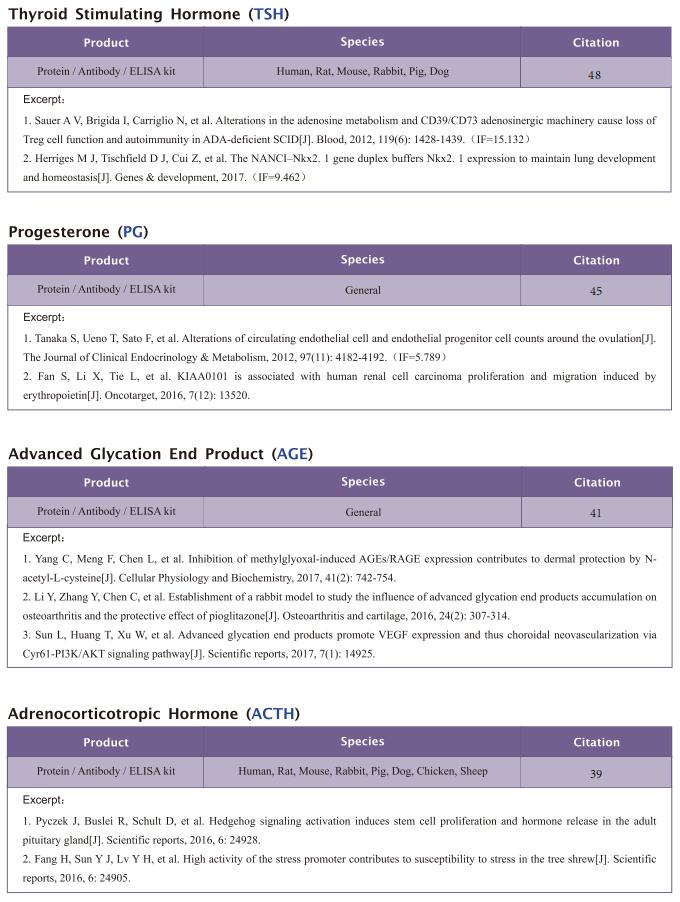
Famsin, a novel gut-secreted hormone, contributes to metabolic adaptations to fasting via binding to its receptor OLFR796

On February 17, 2023, Yiguo Wang, State Key Laboratory of Membrane Biology, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, China, and his team published a paper titled “Famsin, a novel gut-secreted hormone, contributes to metabolic adaptations to fasting via binding to its receptor OLFR796” in Cell Research. Their results demonstrate that communication between the intestine and other organs by a famsin-OLFR796 signaling axis is critical for metabolic adaptations to fasting.

The kits [High Sensitive ELISA Kit for Fibroblast Growth Factor 15 (FGF15), HEL154Mu] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.

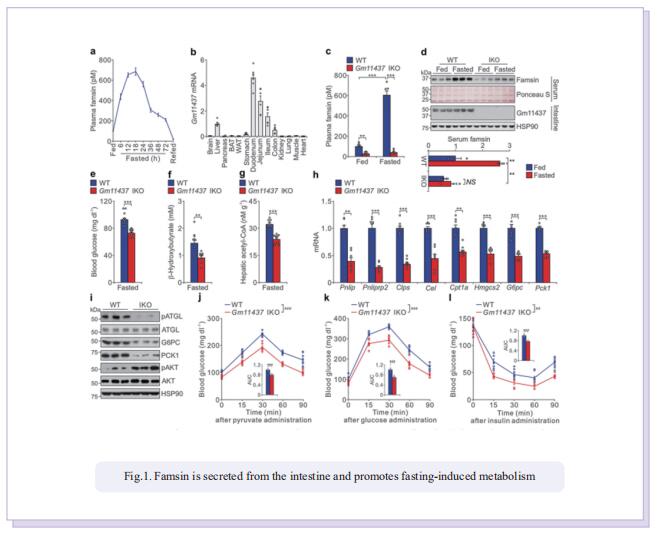
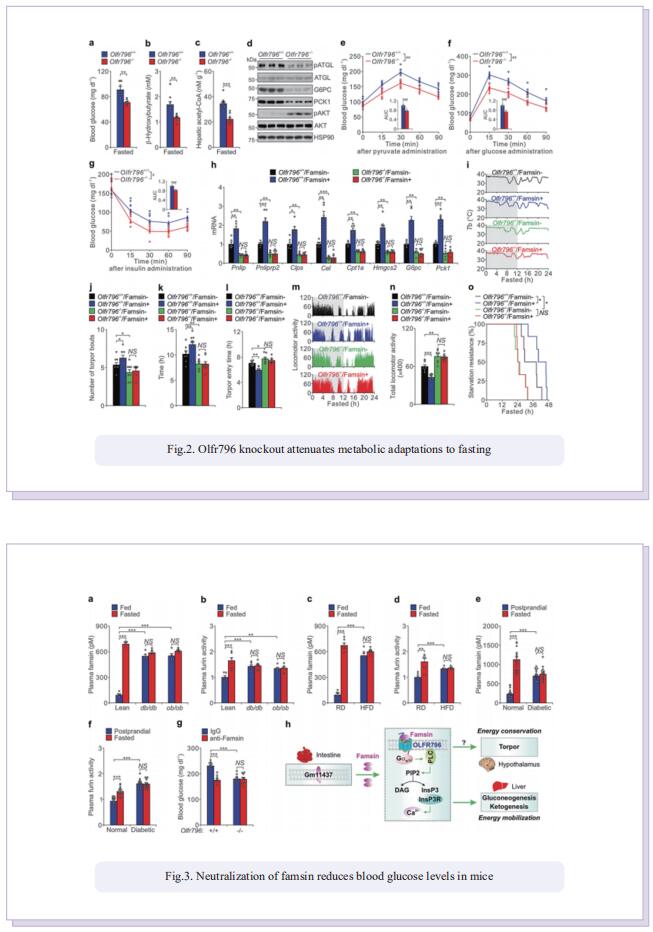
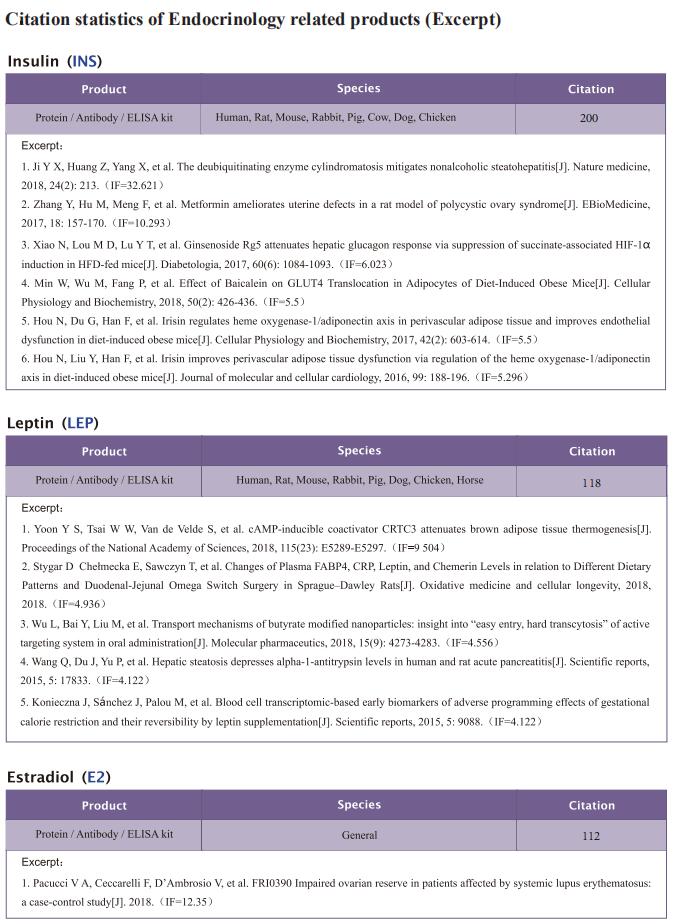
The intestine is responsible for nutrient absorption and orchestrates metabolism in different organs during feeding, a process which is partly controlled by intestine-derived hormones. However, it is unclear whether the intestine plays an important role in metabolism during fasting. Here we have identified a novel hormone, famsin, which is secreted from the intestine and promotes metabolic adaptations to fasting. Mechanistically, famsin is shed from a single-pass transmembrane protein, Gm11437, during fasting and then binds OLFR796, an olfactory receptor, to activate intracellular calcium mobilization. This famsin-OLFR796 signaling axis promotes gluconeogenesis and ketogenesis for energy mobilization, and torpor for energy conservation during fasting. In addition, neutralization of famsin by an antibody improves blood glucose profiles in diabetic models, which identifies famsin as a potential therapeutic target for treating diabetes. Therefore, our results demonstrate that communication between the intestine and other organs by a famsin-OLFR796 signaling axis is critical for metabolic adaptations to fasting.