New discoveries about potential treatments for brain injury
Central nervous system(CNS) injury is one of the leading causes of death and disability. Although the causes of injury are diverse, the common end result is substantial neuronal damage, or neuronal loss, in the affected region. This is thought to underlie the cognitive, sensorimotor function and personality changes typically seen in patients. To date, drug treatments adopting a ‘neuro-centric’ approach have failed to deliver notable clinical benefits for the treatment of CNS injury. Recently, a number of literatures have reported potential therapeutic methods for brain injury, which may help improve the prognosis of patients with brain injury.
1. Astrocyte-targeted gene delivery of IL-2 specifically increases brain-resident Treg cell numbers and protects against pathological neuroinflammation
IL-2 is a high-potential immune-modulating biologic, based on its capacity to support the survival and proliferation of regulatory T (Treg) cells. In injury-driven neuroinflammation, such as stroke or traumatic brain injury, the systemic Treg cell depletion typically used to assess function also drives massive peripheral inflammation, with potential pathological consequences. Adrian Liston, VIB-KU Leuven Center for Brain & Disease Research, Belgium, and his team identified brain IL-2 levels as a limiting factor for brain-resident Treg cells[1]. They developed a gene-delivery approach for astrocytes, with a small-molecule on-switch to allow temporal control, and enhanced production in reactive astrocytes to spatially direct delivery to inflammatory sites. Mice with brain-specific IL-2 delivery were protected in traumatic brain injury, stroke and multiple sclerosis models(Fig.1), without impacting the peripheral immune system. These results validate brain-specific IL-2 gene delivery as effective protection against neuroinflammation, and provide a versatile platform for delivery of diverse biologics to neuroinflammatory patients.
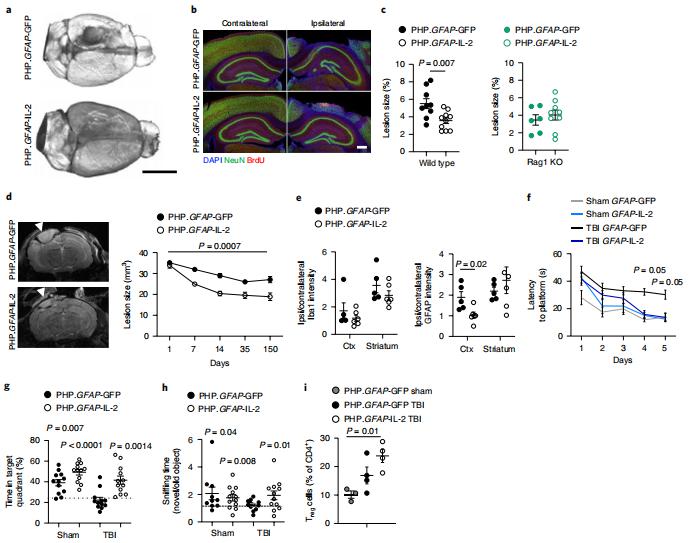
Fig.1 Gene delivery of IL-2 to the brain effectively prevents neurological damage during traumatic brain injury
2. Genetic Deficiency of MicroRNA-15a/16-1 Confers Resistance to Neuropathological Damage and Cognitive Dysfunction in VCID
Chronic cerebral hypoperfusion-derived brain damage contributes to the progression of vascular cognitive impairment and dementia (VCID). Ke-Jie Yin, Veterans Affairs Pittsburgh Healthcare System, USA, and his team attempted to determine the regulatory role of Mir-15A /16-1 in VCID[2]. It is found that miR-15a/16-1 knockout (KO) mice exhibit less cognitive and sensorimotor deficits following VCID. Genetic deficiency of miR-15a/16-1 in VCID mice also mitigate myelin degeneration, axonal injury, and neuronal loss. Mechanistically, miR-15a/16-1 binds to the 3’-UTR of AKT3 and IL-10RA. Genetic deletion of miR-15a/16-1 increases AKT3 and IL-10RA expression in VCID brains, and intranasal delivery of AKT3 and IL-10RA siRNA-loaded nanoparticles partially reduce brain protection and cognitive recovery in miR-15a/16-1 KO mice after VCID(Fig.2). In conclusion, the miR-15a/16-1-IL/10RA/AKT3 axis plays a critical role in regulating vascular brain damage and cognitive decline after VCID. Targeting miR-15a/16-1 is a novel therapeutic approach for the treatment of VCID.
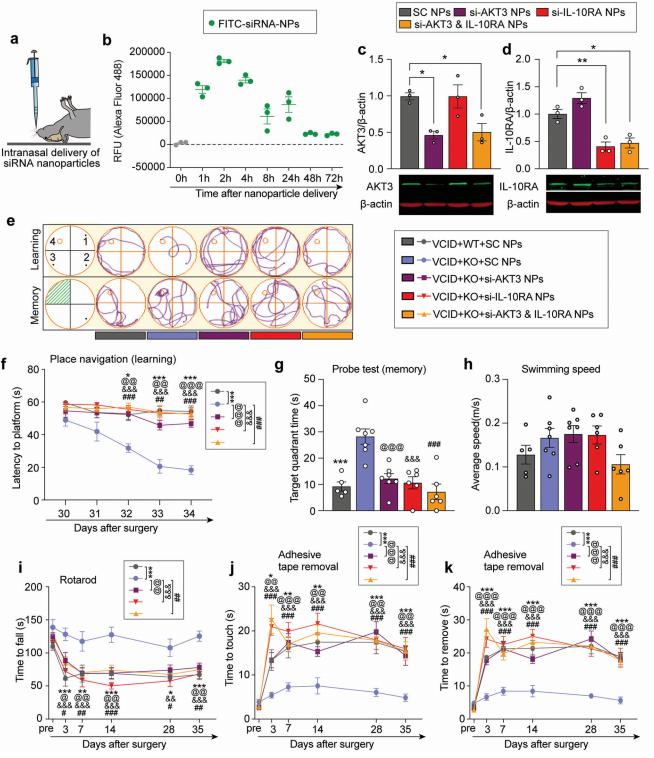
Fig.2 Knockdown of cerebral AKT3 and IL-10RA partially abolishes long-term neurobehavioral improvements in miR-15a/16-1 KO mice after VCID
3. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging
Adult hippocampal neurogenesis is an evolutionarily conserved process that provides a particular type of structural plasticity to the brain, allowing life-long flexibility that supports effective learning and memory. The broad range of effects of exercise across the body makes it likely that systemically released factors in the blood could serve as systemic mediators of the neurogenesis-promoting effect. Tara L. Walker, Queensland Brain Institute, The University of Queensland, Australia, and his team sought to identify systemic mechanisms by which exercise regulates adult hippocampal neurogenesis[3]. They proposed that this is mediated by the exercise-induced systemic release of the antioxidant selenium transport protein, selenoprotein P (SEPP1). Using knockout mouse models, they confirmed that SEPP1 and its receptor low-density lipoprotein receptor-related protein 8 (LRP8) are required for the exercise-induced increase in adult hippocampal neurogenesis. In vivo selenium infusion increased hippocampal neural precursor cell (NPC) proliferation and adult neurogenesis(Fig.3). Mimicking the effect of exercise through dietary selenium supplementation restored neurogenesis and reversed the cognitive decline associated with aging and hippocampal injury, suggesting potential therapeutic relevance.
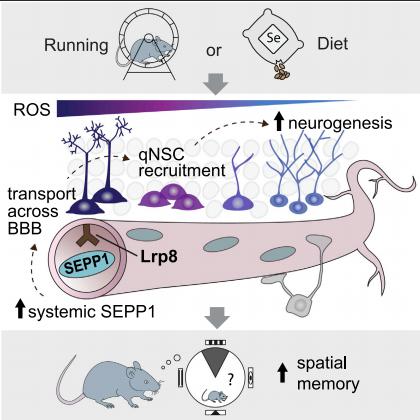
Fig.3 Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging
References
[1]Yshii L, Pasciuto E, Bielefeld P, et al. Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation [J]. Nat Immunol. 2022, 10.1038/s41590-022-01208-z. (IF=25.606)
[2]Zhou C, Sun P, Xu Y, et al. Genetic Deficiency of MicroRNA-15a/16-1 Confers Resistance to Neuropathological Damage and Cognitive Dysfunction in Experimental Vascular Cognitive Impairment and Dementia [J]. Adv Sci (Weinh). 2022, e2104986. (IF=16.806)
[3]Leiter O, Zhuo Z, Rust R, et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging [J]. Cell Metab. 2022, 34(3):408-423.e8. (IF=27.287)
Cloud-Clone not only provides animal models of hypoxic-ischemic brain injury and closed mechanical brain injury, but also has various commonly used brain injury detection indicators and the above-mentioned IL-2, AKT3, IL-10RA, SEPP1, LRP8 protein related products, which can can help the majority of scientific researchers to carry out research related to the treatment of brain injury.

