New discovery of potential treatment for glioblastoma
Brain tumors are among the most devastating forms of cancerand glioblastoma (GBM) represents the most aggressive and lethalform of the disease. GBM is characterized by a poor prognosis, an extremely high mortality rate, and a high tendency for recurrence. Despite advances in surgery, radiation, and chemotherapy, the median survival time of GBM is only 15–16 months, and the 5-year overall survival rate is less than 5%. Thus, there is an urgent need to develop new effective therapeutic strategies for treating GBM. Recently, a number of studies have reported new advances in GBM research, which may help improve the clinical outcomes of GBM.
1. M1-macrophage extracellular vesicles for effective accumulation in GBM and strong synergistic therapeutic effects
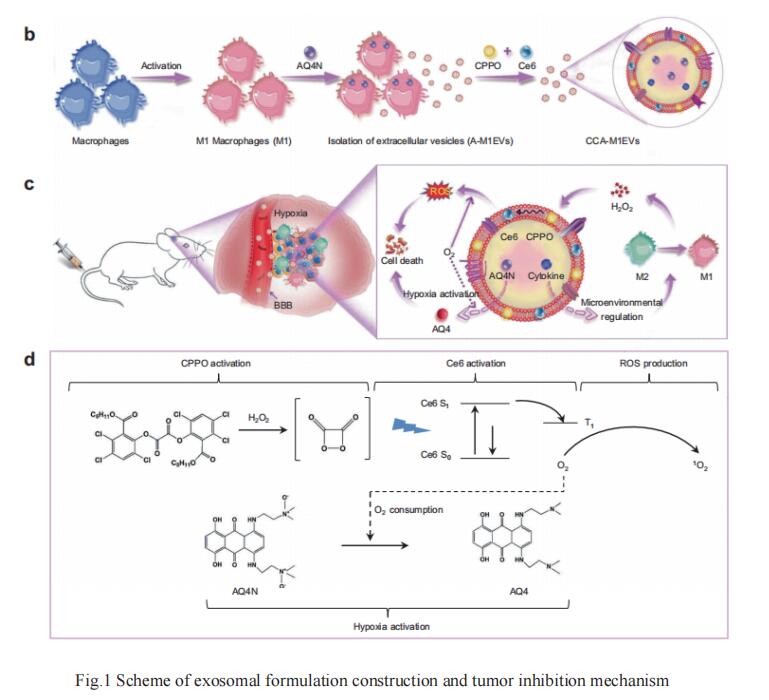
Wei Wei, State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, and his team reported the development of M1-like macrophage-derived extracellular vesicles (M1EVs) as a drug delivery system for GBM treatment [1]. M1-like macrophages were incubated with the inactivated chemotherapy agent banoxantrone (AQ4N, A) followed by release and isolation of M1EVs carrying AQ4N in the inner core (A-M1EVs). These drug-loaded EVs were subsequently elaborated with hydrophobic bis(2,4,5-trichloro-6-carbopentoxyphenyl) oxalate (CPPO, C) and chlorin e6 (Ce6, C) in their membranes to obtain CCA-M1EVs. After accumulating in GBM tumors, CCA-M1EVs induced M2-to-M1 polarization for tumor microenvironment immunomodulation, leading to increased H2O2 levels. Furthermore, chemical energy was produced by the reaction between H2O2 and CPPO, and this energy could be used for Ce6 activation to generate large amounts of reactive oxygen species to achieve chemiexcited photodynamic therapy (CDT). As this reaction consumed oxygen, aggravation of tumor hypoxia also led to the conversion of non-toxic AQ4N to toxic AQ4 for chemotherapy. Therefore, CCA-M1EVs achieved synergistic immunomodulation, CDT, and hypoxia-activated chemotherapy in GBM to exert a potent therapeutic effect (Fig.1). Finally, they demonstrated the excellent effect of CCA-M1EVs against GBM in cell-derived xenograft and patient-derived xenograft models, underscoring the strong potential of our highly flexible M1EVs system to support multi-modal therapies for difficult-to-treat GBM.
2. Neisseria meningitidis Opca Protein/MnO2 Hybrid Nanoparticles for Overcoming the Blood–Brain Barrier to Treat GBM
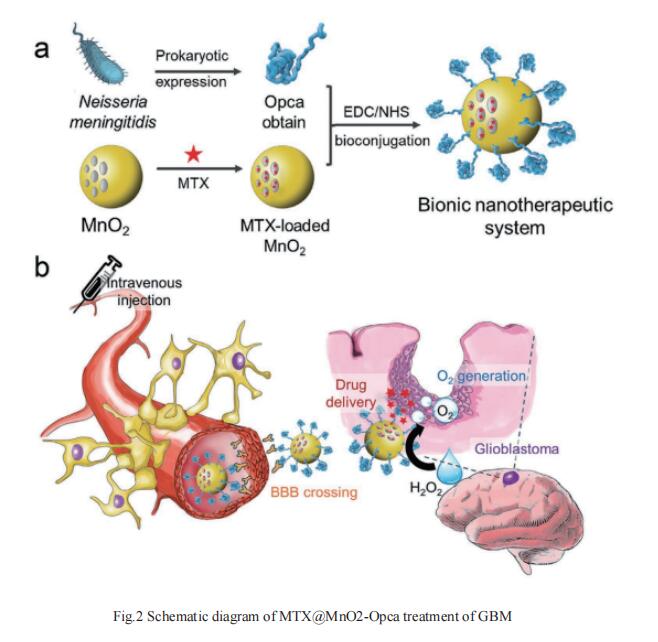
The major hurdle in GBM therapy is the low efficacy of drugs crossing the blood–brain barrier (BBB). Neisseria meningitidis is known to specifically enrich in the central nervous system through the guidance of an outer membrane invasion protein named Opca. By loading a chemotherapeutic drug methotrexate (MTX) in hollow manganese dioxide (MnO2) nanoparticles with surface modification of the Opca protein of Neisseria meningitidis, Xian-Zheng Zhang, Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, China, and his team demonstrated a bionic nanotherapeutic system (MTX@MnO2-Opca) effectively overcomed the BBB[2]. The presence of the Opca protein enables the drug to cross the BBB and penetrate into tumor tissues. After accumulating in GBM, the nanotherapeutic system catalyzes the decomposition of excess H2O2 in the tumor tissue and thereby generates O2, which alleviates tumor hypoxia and enhances the effect of chemotherapy in the treatment of GBM(Fig.2). This bionic nanotherapeutic system may exhibit great potential in the treatment of GBM.
3. SOX2 induces GBM cell stemness and tumor propagation by repressing TET2 and deregulating 5hmC and 5mC DNA modifications
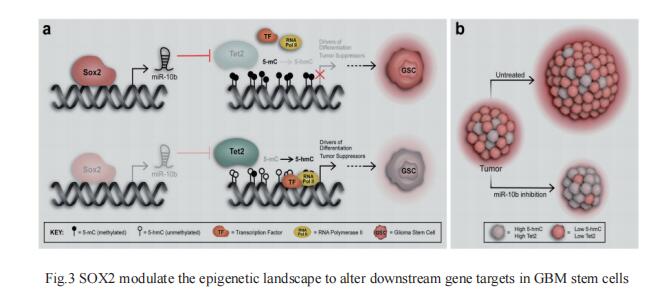
DNA methylation is a reversible process catalyzed by the ten–eleven translocation (TET) family of enzymes (TET1, TET2, TET3) that convert 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC). Altered patterns of 5hmC and 5mC are widely reported in human cancers and loss of 5hmC correlates with poor prognosis. John Laterra, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, USA, and his team showed that TET2 loss associates with GBM stem cells and correlates with poor survival of GBM patients[3]. They further identified a SOX2:miR-10b-5p:TET2 axisthat represses TET2 expression, represses 5hmC, increases 5mC levels, and induces GBM cell stemness and tumor-propagating potential(Fig.3). In vivo delivery of a miR-10b-5p inhibitor that normalizes TET2 expression and 5hmC levels inhibits tumor growth and prolongs survival of animals bearing pre-established orthotopic GBM xenografts. These findings highlight the importance of TET2 and 5hmC loss in SOX2-driven oncogenesis and their potential for therapeutic targeting.
4. Transcription Elongation Machinery Potentiates Immunotherapy in GBM Stem Cells
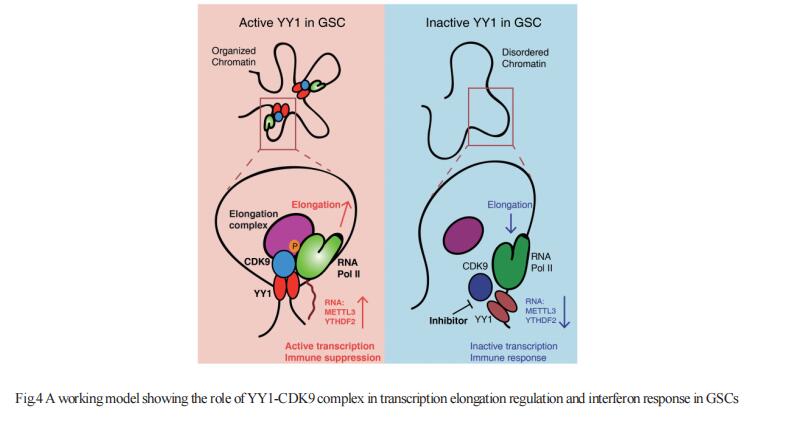
GBM is the most lethal primary brain cancer characterized by therapeutic resistance, which is promoted by GBM stem cells (GSCs). Jeremy N. Rich, UPMC Hillman Cancer Center, USA, and his team interrogated gene expression and whole genome CRISPR/Cas9 screening in a large panel of patient-derived GSCs, differentiated GBM cells (DGCs), and neural stem cells (NSCs) to identify master regulators of GSC stemness, revealing an essential transcription state with increased RNA polymerase II-mediated transcription[4]. The YY1 and transcriptional CDK9 complex was essential for GSC survival and maintenance in vitro and in vivo. YY1 interacted with CDK9 to regulate transcription elongation in GSCs. Genetic or pharmacological targeting of YY1-CDK9 complex elicited RNA m6A modification-dependent interferon responses, reduced regulatory T cell infiltration, and augmented efficacy of immune checkpoint therapy in GBM(Fig.4). Collectively, these results suggest that YY1-CDK9 transcription elongation complex defines a targetable cell state with active transcription, suppressed interferon responses, and immunotherapy resistance in GBM.
References
[1]Wang X, Ding H, Li Z, et al. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects[J]. Signal Transduct Target Ther. 2022;7(1):74.(IF=18.187)
[2]Dong CY, Huang QX, Cheng H, et al. Neisseria meningitidis Opca Protein/MnO2 Hybrid Nanoparticles for Overcoming the Blood-Brain Barrier to Treat Glioblastoma[J]. Adv Mater. 2022;34(12):e2109213.(IF=30.849)
[3]Lopez-Bertoni H, Johnson A, Rui Y, et al. Sox2 induces glioblastoma cell stemness and tumor propagation by repressing TET2 and deregulating 5hmC and 5mC DNA modifications[J]. Signal Transduct Target Ther. 2022;7(1):37.(IF=18.187)
[4]Qiu Z, Zhao L, Shen JZ, et al. Transcription Elongation Machinery Is a Druggable Dependency and Potentiates Immunotherapy in Glioblastoma Stem Cells[J]. Cancer Discov. 2022;12(2):502-521. (IF=39.397)
Cloud-Clone can not only provide a variety of experimental tumor animal models, including tumor transplantation animal model, spontaneous tumor animal model, induced tumor animal model, tumor metastasis animal model, covering common tumor research. We also have all kinds of cancer detection indicators and the above SOX2, YY1, CDK9 related products, which can help the majority of researchers to carry out cancer related research.