New findings from schizophrenia gene study
Schizophrenia is a severe psychiatric disorder with signs and symptoms that include hallucinations, delusions, disorganized speech and behaviour, diminished emotional expression, social withdrawal and cognitive impairment, typically manifests in late adolescence or early adulthood. Existing therapies largely address primarily positive symptoms (hallucinations and delusions), and the response to existing antipsychotic medications is highly variable, with approximately 30% of patients classified as treatment resistant. The lack of progress in therapeutic development is in part a consequence of the limited understanding of the molecular aetiology of psychiatric disorders.Recently, a number of studies on schizophrenia genes have been reported, providing help for further understanding the pathogenesis of the disease.
1. Mapping genomic loci implicates genes and synaptic biology in schizophrenia
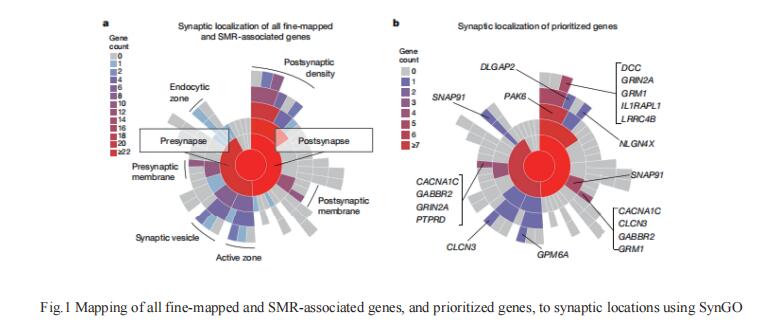
Schizophrenia has a heritability of 60–80%, much of which is attributable to common risk alleles. Stephan Ripke, Department of Psychiatry and Psychotherapy, Charité - Universitätsmedizin, Germany, James T. R. Walters and Michael C. O’Donovan of MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, UK, and their team reported common variant associations at 287 distinct genomic loci in a two-stage genome-wide association study of up to 76,755 individuals with schizophrenia and 243,649 control individuals[1]. Enrichment of common variant associations was restricted to genes that are expressed in neurons of the central nervous system—both excitatory and inhibitory—and that have roles in fundamental biological processes related to neuronal function. This indicates that neurons are the most important site of pathology in schizophrenia. Fine-mapped candidates were enriched for genes associated with rare disruptive coding variants in people with schizophrenia, including the glutamate receptor subunit GRIN2A, and were also enriched for genes implicated by such variants in neurodevelopmental disorders(Fig.1). The study identify biological processes relevant to schizophrenia pathophysiology, show convergence of common and rare variant associations in schizophrenia and neurodevelopmental disorders, and provide a resource of prioritized genes and variants to advance mechanistic studies.
2. Rare coding variants in ten genes confer substantial risk for schizophrenia
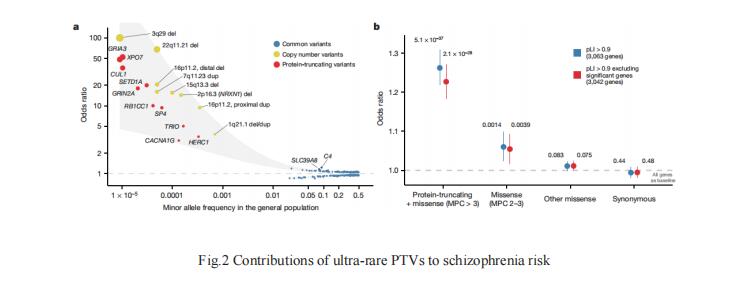
Rare coding variation has historically provided the most direct connections between gene function and disease pathogenesis. Benjamin M. Neale and Mark J. Daly of Analytic and Translational Genetics Unit, Department of Medicine, Massachusetts General Hospital, Boston, USA, and their team implicate ultra-rare coding variants (URVs) in 10 genes as conferring substantial risk for schizophrenia by meta-analysing the whole exomes of 24,248 schizophrenia cases and 97,322 controls[2]. These genes have the greatest expression in central nervous system neurons and have diverse molecular functions that include the formation, structure and function of the synapse. Among them, the association of protein-truncating variants(PTVs) in the NMDA receptor subunit GRIN2A with schizophrenia risk provides genetic support for the dysregulation of glutamatergic signalling as a possible mechanism of disease(Fig.2). A natural dose–response curve occurs at this gene, in which common regulatory variants modestly influence disease risk while PTVs and predicted damaging missense variants increase risk more substantially. These emerging genetic findings will serve in part to direct and motivate mechanistic studies that begin to unravel disease biology.
3. Chromatin domain alterations linked to 3D genome organization in schizophrenia
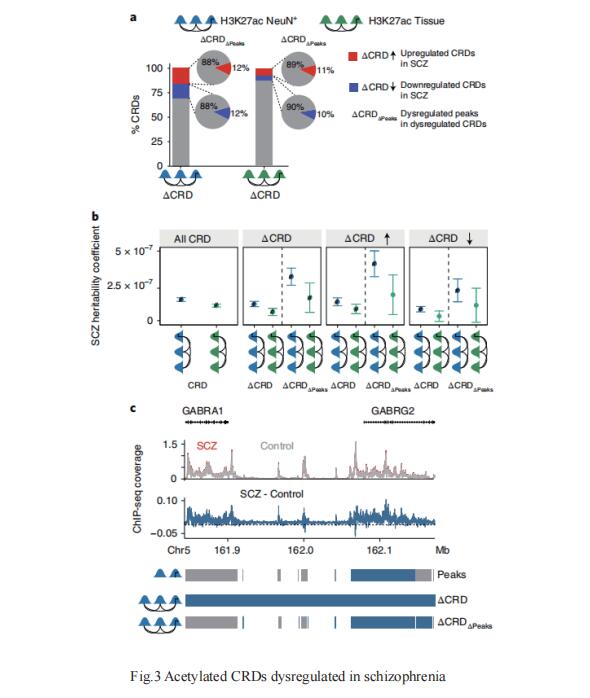
Chromosomal organization, scaling from the 147-base pair (bp) nucleosome to megabase-ranging domains encompassing multiple transcriptional units, including heritability loci for psychiatric traits, remains largely unexplored in the human brain. Panos Roussos and Schahram Akbarian, Icahn School of Medicine at Mount Sinai, New York, NY, USA, and their team constructed promoter- and enhancer-enriched nucleosomal histone modification landscapes for adult prefrontal cortex from H3-lysine 27 acetylation and H3-lysine 4 trimethylation profiles, generated from 388 controls and 351 individuals diagnosed with schizophrenia or bipolar disorder[3]. They mapped thousands of cis-regulatory domains (CRDs), revealing fine-grained, 104–106-bp chromosomal organization, firmly integrated into Hi-C topologically associating domain stratification by open/repressive chromosomal environments and nuclear topography. Large clusters of hyper-acetylated CRDs were enriched for schizophrenia heritability(Fig.3), with prominent representation of regulatory sequences governing fetal development and glutamatergic neuron signaling. Therefore, schizophrenia brains show coordinated dysregulation of risk-associated regulatory sequences assembled into kilobase- to megabase-scaling chromosomal domains.
References
[1]Trubetskoy V, Pardiñas AF, Qi T, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia [J]. Nature. 2022;10.1038/s41586-022-04434-5.(IF=49.962)
[2]Singh T, Poterba T, Curtis D, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia [J]. Nature. 2022;10.1038/s41586-022-04556-w. (IF=49.962)
[3]Girdhar K, Hoffman GE, Bendl J, et al. Chromatin domain alterations linked to 3D genome organization in a large cohort of schizophrenia and bipolar disorder brains[J]. Nat Neurosci. 2022;25(4):474-483. (IF=24.884)
Cloud-Clone can not only provide a variety of animal models of mental diseases, including schizophrenia, chronic stress depression, anxiety, etc., covering common mental diseases research. We can also provide mental disease-related index detection products and the above-mentioned GRIN2A protein related products, which can help the majority of scientific researchers to carry out mental diseases related research.