New findings in research on endometriosis
Endometriosis, characterized by the growth of endometrium-like tissue outside the uterus, is a common gynecological disorder in women of childbearing age and can cause pain and infertility. Its pathophysiology remains largely unknown, limiting diagnosis and treatment. Current treatment options are limited to pain-relieving hormonal drugs or surgical treatments such as hysterectomy or removal of lesions. However, the treatment has limited effectiveness and side effects. Recently, several studies have reported on endometriosis, which may provide help for the early diagnosis and treatment of endometriosis.
1. A long-acting anti–IL-8 antibody improves inflammation and fibrosis in endometriosis
Studies of human endometriotic samples have shown that that the progression of endometriosis was associated with the development of inflammation and fibrosis. In addition, IL-8 expression was highly up-regulated in endometriotic tissues and closely correlated with disease progression. Ryo Konno, Department of Obstetrics and Gynecology, Jichi Medical University Saitama Medical Center, Japan, and his team created a long-acting recycling antibody against IL-8 (AMY109) and evaluated its clinical potency[1]. They analyzed the lesions in cynomolgus monkeys that spontaneously developed endometriosis and in a surgically induced endometriosis monkey model. Both spontaneously developed and surgically induced endometriotic lesions demonstrated pathophysiology that was highly similar to that of human endometriosis. Once-a-month subcutaneous injection of AMY109 to monkeys with surgically induced endometriosis reduced the volume of nodular lesions, lowered the Revised American Society for Reproductive Medicine score as modified for monkeys, and ameliorated fibrosis and adhesions. In addition, experiments using cells derived from human endometriosis revealed that AMY109 inhibited the recruitment of neutrophils to endometriotic lesions and the production of monocyte chemoattractant protein-1 from neutrophils (Fig.1). Thus, AMY109 may represent a disease-modifying therapy for patients with endometriosis.
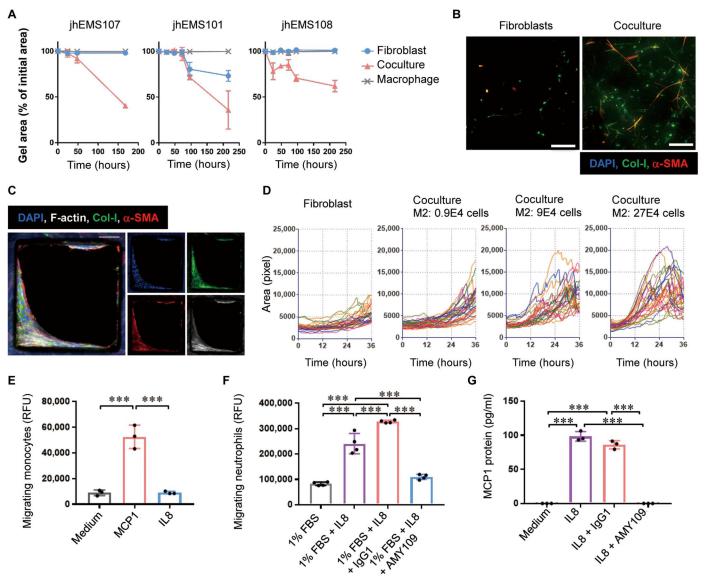
Fig.1 Effect of AMY109 on fibrosis and inflammatory cells
2. Single cell analysis of endometriosis reveals a coordinated transcriptional program driving immunotolerance and angiogenesis across eutopic and ectopic tissues
Elise T. Courtois, The Jackson Laboratory for Genomic Medicine, USA, and his team characterized peritoneal and ovarian lesions at single-cell transcriptome resolution and compared to matched eutopic endometrium, control endometrium, and organoids derived from these tissues, generating data on over 100,000 cells across 12 individuals[2]. They spatially localized many of the cell types using imaging mass cytometry. They identified a perivascular mural cell unique to the peritoneal lesions with dual roles in angiogenesis promotion and immune cell trafficking (Fig.2). They defined an immunotolerant peritoneal niche, fundamental differences in eutopic endometrium and between lesions microenvironments, and a novel progenitor-like epithelial cell subpopulation. Altogether, this study provides a holistic view of the endometriosis microenvironment representing the first comprehensive cell atlas of the disease, essential information for advancing therapeutics and diagnostics.
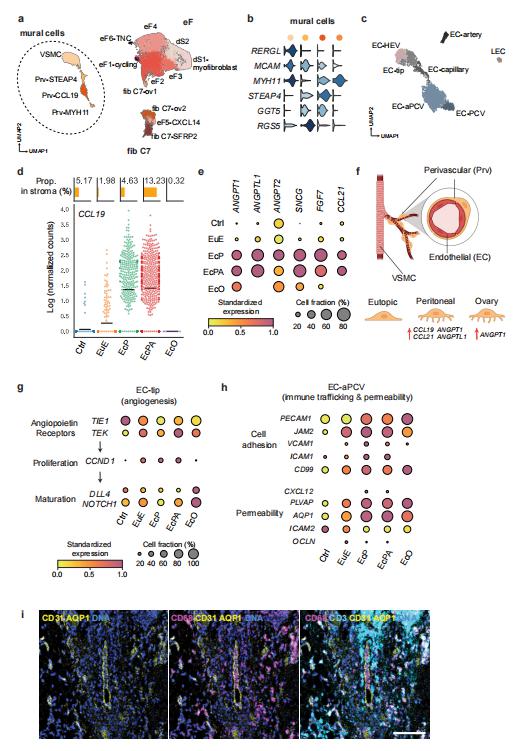
Fig.2 Role of Stromal cell diversity in angiogenesis and immune trafficking in endometriosis lesions
3. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions
A genome-wide association study meta-analysis conducted by Nilufer Rahmioglu, Wellcome Centre for Human Genetics, University of Oxford, UK, and his team, including 60,674 cases and 701,926 controls of European and East Asian descent, identified 42 genome-wide significant loci comprising 49 distinct association signals[3]. Identified signals explained up to 5.01% of disease variance and regulated expression or methylation of genes in endometrium and blood, many of which were associated with pain perception/maintenance (SRP14/BMF, GDAP1, MLLT10, BSN, NGF). We observed significant genetic correlations between endometriosis and 11 pain-conditions including migraine, back, and multisite chronic pain (MCP), as well as inflammatory conditions including asthma and osteoarthritis (Fig.3). Multi-trait genetic analyses identified substantial sharing of variants associated with endometriosis and MCP/migraine. Targeted investigations of genetically regulated mechanisms shared between endometriosis and other pain-conditions are needed to aid the development of new treatments and facilitate early symptomatic intervention.
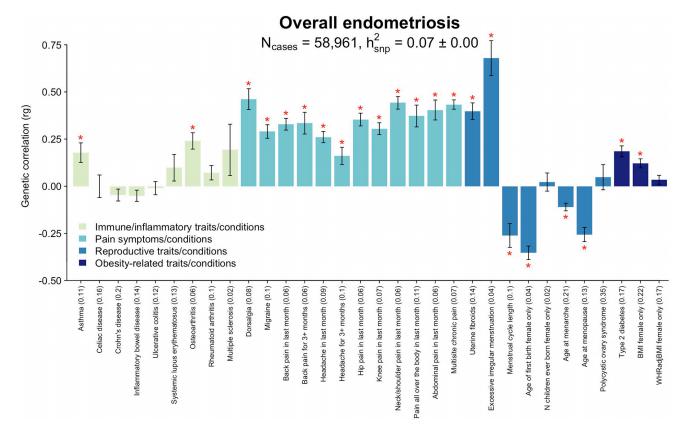
Fig.3 Genetic correlation between endometriosis and 32 immune/inflammatory, pain, reproductive, and metabolic traits/conditions
References
[1]Nishimoto-Kakiuchi A, Sato I, Nakano K, et al. A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis [J]. Sci Transl Med. 2023;15(684):eabq5858. (IF=19.319)
[2]Tan Y, Flynn WF, Sivajothi S, et al. Single-cell analysis of endometriosis reveals a coordinated transcriptional programme driving immunotolerance and angiogenesis across eutopic and ectopic tissues [J]. Nat Cell Biol. 2022;24(8):1306-1318. (IF=28.213)
[3]Rahmioglu N, Mortlock S, Ghiasi M, et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions [J]. Nat Genet. 2023;55(3):423-436. (IF=41.307)
Cloud-Clone can not only provide a variety of reproductive system disease models, including endometriosis, spontaneous abortion, polycystic ovaries, tubal obstruction, uterine fibroids, etc., covering common reproductive system disease research. There are also various endocrine related indicators and the above IL-8, CCL19 and other detection products, which can help the majority of scientific researchers to conduct research on reproductive system diseases.