New findings in the repair of heart injury
Through normal wear and tear, our tissues experience regular cell loss that is countered by replenishment mechanisms. Cardiomyocytes, the contractile cells of the heart, display an estimated turnover rate of 0.3 to 1% per year. Due to this low turnover rate, each of us will die with many or most of the cardiomyocytes we had at birth. In addition to longevity, cardiomyocytes possess brute strength and resilience. Their kryptonite is coronary artery occlusion and tissue ischemia, which can locally devastate the cardiomyocyte population and cause myocardial infarction (MI) with resultant scarring, along with nonischemic causes of muscle loss. Unfortunately, a scarred heart is much more likely to fail over time, and further lead to death. Recently, a number of studies have reported new findings of myocardial repair, which may provide help for the treatment of myocardial injury.
1. Dual human iPSC-derived cardiac lineage cell-seeding extracellular matrix patches promote regeneration and long-term repair of infarcted hearts
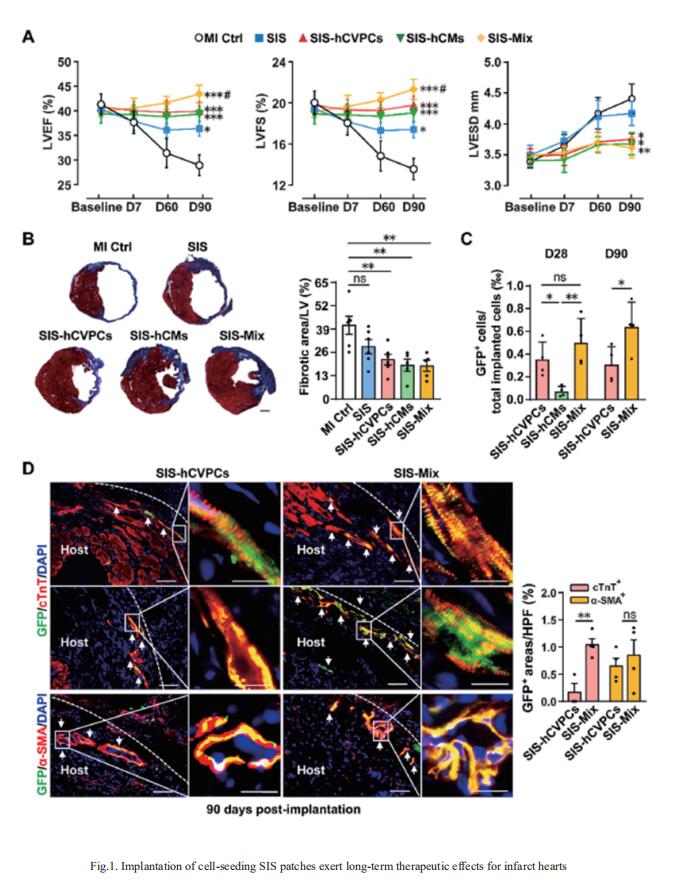
Human pluripotent stem cell-derived cardiovascular progenitor cells (hCVPCs) and cardiomyocytes (hCMs) possess therapeutic potential for infarcted hearts; however, their efficacy needs to be enhanced. Huang-Tian Yang, Translational Medical Center for Stem Cell Therapy & Institute for Heart Failure and Regenerative Medicine, Shanghai East Hospital, School of Medicine, Tongji University, China, and his team tested the hypotheses that the combination of decellularized porcine small intestinal submucosal extracellular matrix (SIS-ECM) with hCVPCs, hCMs, or dual of them (Mix, 1:1) could provide better therapeutic effects than the SIS alone, and dual hCVPCs with hCMs would exert synergic effects in cardiac repair[1]. The data showed that the SIS patch well supported the growth of hCVPCs and hCMs. Epicardially implanted SIS-hCVPC, SIS-hCM, or SIS-Mix patches at 7-day post-myocardial infarction significantly ameliorated functional worsening, ventricular dilation and scar formation at 28- and 90-day post-implantation in C57/B6 mice. Further, the SIS-cell patches stimulated cardiomyocyte proliferation via paracrine action (Fig.1). Notably, the SIS-Mix had better improvements in cardiac function and structure, engraftments, and cardiomyocyte proliferation. These results suggest that the implantation of dual hCVPC- and hCM-seeding SIS patches exhibits synergistic and complementary effects in promoting infarct repair, which might be a promising therapeutic approach for ischemic hearts disease.
2. Inhibition of Eicosanoid Degradation Mitigates Fibrosis of the Heart
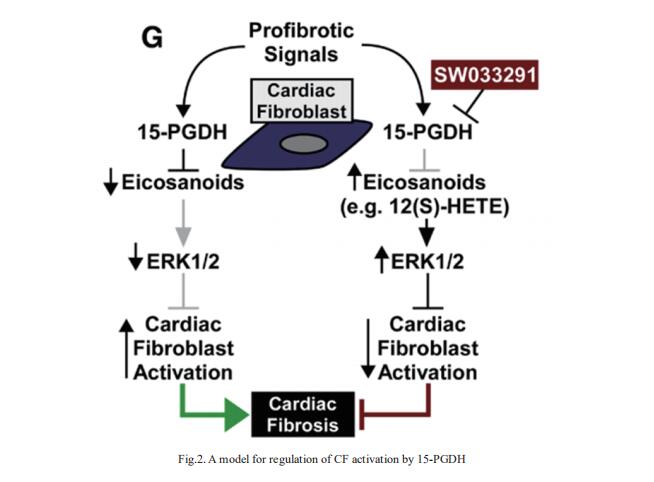
Organ fibrosis due to excessive production of extracellular matrix by resident fibroblasts is estimated to contribute to >45% of deaths, including those due to cardiovascular diseases such as heart failure. Timothy A. McKinsey, Department of Medicine, Division of Cardiology, Consortium for Fibrosis Research & Translation, University of Colorado Anschutz Medical Campus, Aurora, and his team screened for small molecule inhibitors with a common ability to suppress activation of fibroblasts across organ systems[2]. SW033291, which inhibits the eicosanoid-degrading enzyme, 15-hydroxyprostaglandin dehydrogenase (15-PGDH), dose-dependently inhibited transforming growth factor-β1–induced expression of activation markers (eg, α-smooth muscle actin and periostin) in adult rat ventricular fibroblasts and normal human cardiac fibroblasts (CFs), and reduced contractile capacity of the cells. The 15-PGDH inhibitor also reversed constitutive activation of fibroblasts obtained from explanted hearts from patients with heart failure. SW033291 blocked cardiac fibrosis induced by angiotensin II infusion and ameliorated diastolic dysfunction in an alternative model of systemic hypertension driven by combined uninephrectomy and deoxycorticosterone acetate administration. Mechanistically, SW033291-mediated stimulation of extracellular signal-regulated kinase 1/2 mitogenactivated protein kinase signaling was required for the compound to block CF activation (Fig.2). In the heart, they propose that 15-PGDH inhibition triggers CF-derived autocrine/paracrine signaling by eicosanoids, including 12(S)-hydroxyeicosatetraenoic acid, to stimulate extracellular signal-regulated kinase 1/2 and block conversion of fibroblasts into activated cells that secrete excessive amounts of extracellular matrix and contribute to heart failure pathogenesis.
3. Interplay between calcium and sarcomeres directs cardiomyocyte maturation during regeneration
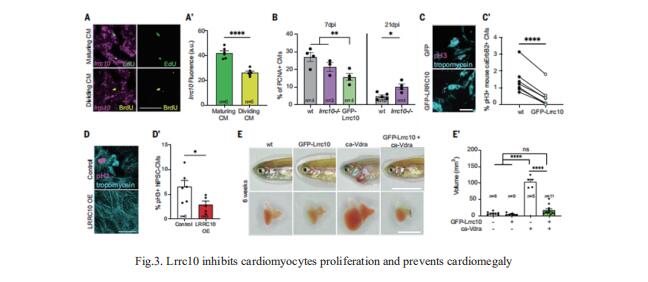
Zebrafish hearts can regenerate by replacing damaged tissue with new cardiomyocytes. Although the steps leading up to the proliferation of surviving cardiomyocytes have been extensively studied, little is known about the mechanisms that control proliferation and redifferentiation to a mature state. Jeroen Bakkers, Hubrecht Institute-KNAW and University Medical Center Utrecht, Utrecht, Netherlands, and his team found that the cardiac dyad, a structure that regulates calcium handling and excitationcontraction coupling, played a key role in the redifferentiation process[3]. A component of the cardiac dyad called leucine-rich repeat-containing 10 (Lrrc10) acted as a negative regulator of proliferation, prevented cardiomegaly, and induced redifferentiation (Fig.3). They found that its function was conserved in mammalian cardiomyocytes. This study highlights the importance of the underlying mechanisms required for heart regeneration and their application to the generation of fully functional cardiomyocytes.
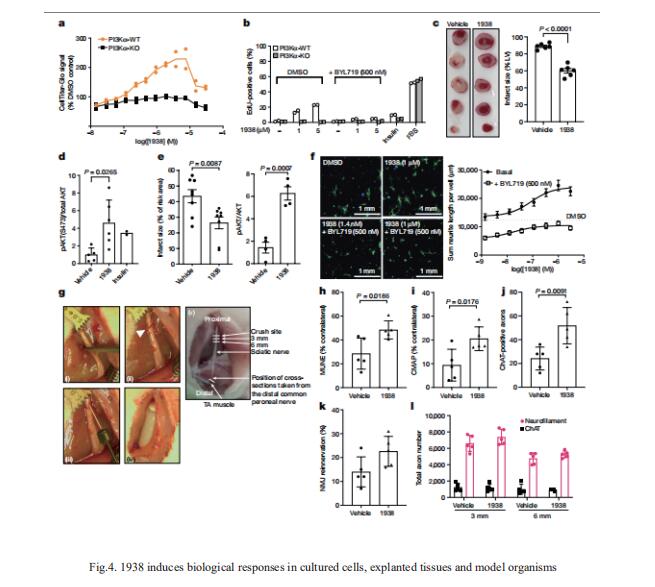
4. A small-molecule PI3Kα activator for cardioprotection and neuroregeneration
Harnessing the potential beneficial effects of kinase signalling through the generation of direct kinase activators remains an underexplored area of drug development. Bart Vanhaesebroeck, Cell Signalling, Cancer Institute, University College London, UK, and his team reported the discovery of UCL-TRO-1938 (referred to as 1938 hereon), a small-molecule activator of the PI3Kα isoform, a crucial effector of growth factor signalling[4]. This compound is selective for PI3Kα over other PI3K isoforms and multiple protein and lipid kinases. It transiently activates PI3K signalling in all rodent and human cells tested, resulting in cellular responses such as proliferation and neurite outgrowth. In rodent models, acute treatment with 1938 provides cardioprotection from ischaemia–reperfusion injury and, after local administration, enhances nerve regeneration following nerve crush (Fig.4). This study identified a chemical tool to directly probe the PI3Kα signalling pathway and a new approach to modulate PI3K activity, widening the therapeutic potential of targeting these enzymes through short-term activation for tissue protection and regeneration.
References
[1]Jiang Y, Zhang LL, Zhang F, et al. Dual human iPSC-derived cardiac lineage cell-seeding extracellular matrix patches promote regeneration and long-term repair of infarcted hearts. Bioact Mater. 2023;28:206-226. (IF=18.9)
[2]Rubino M, Travers JG, Headrick AL, et al. Inhibition of Eicosanoid Degradation Mitigates Fibrosis of the Heart. Circ Res. 2023;132(1):10-29. (IF=20.1)
[3]Nguyen PD, Gooijers I, Campostrini G, et al. Interplay between calcium and sarcomeres directs cardiomyocyte maturation during regeneration. Science. 2023;380(6646):758-764. (IF=56.9)
[4]Gong GQ, Bilanges B, Allsop B, et al. A small-molecule PI3Kα activator for cardioprotection and neuroregeneration. Nature. 2023;618(7963):159-168. (IF=64.8)
Cloud-Clone can not only provide a variety of cardiovascular system disease models, including myocardial infarction, arrhythmia, myocardial hypertrophy, myocarditis, heart failure, covering common cardiovascular system diseases. We also have various commonly used indicators of cardiovascular system signaling pathways and the above TGFβ1, ERK1/2, PI3K and other related products, which can help the majority of scientific researchers to carry out research on cardiovascular system diseases.