New findings on the mechanism of Crohn's disease
Crohn’s disease is a chronic inflammatory condition that affects the gastrointestinal tract. It can cause lesions from mouth to anus and may result in extraintestinal complications. Crohn's disease might result from a complex interplay between genetic susceptibility, environmental factors, and altered gut microbiota, leading to dysregulated innate and adaptive immune responses. The therapies generally consist of steroids for rapid palliation of symptoms, monoclonal antibodies to IL-12/23 or integrin α4β7, immunomodulators, combination therapies, or surgery. However, these therapies may cause adverse reactions and have limited efficacy. Further exploration of the pathogenesis of Crohn's disease may contribute to the development of more effective and safe treatments.
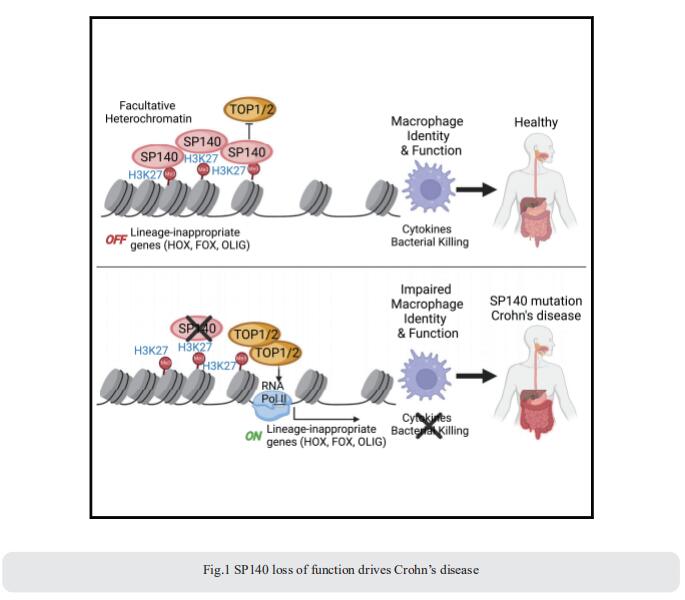
1. Epigenetic reader SP140 loss of function drives Crohn’s disease
How mis-regulated chromatin directly impacts human immune disorders is poorly understood. Speckled Protein 140 (SP140) is an immune-restricted PHD and bromodomain-containing epigenetic ‘‘reader,’’ and SP140 loss-of-function mutations associate with Crohn’s disease. Using a global proteomic strategy, Kate L. Jeffrey, Center for the Study of Inflammatory Bowel Disease, Division of Gastroenterology, Department of Medicine, Massachusetts General Hospital Research Institute, USA, and his team identified SP140 as a repressor of topoisomerases (TOPs) that maintains heterochromatin and macrophage fate[1]. In humans and mice, SP140 loss resulted in unleashed TOP activity, de-repression of developmentally silenced genes, and ultimately defective microbe-inducible macrophage transcriptional programs and bacterial killing that drive intestinal pathology (Fig.1). Pharmacological inhibition of TOP1/2 rescued these defects. Furthermore, exacerbated colitis was restored with TOP1/2 inhibitors in Sp140-/- mice, but not wild-type mice, in vivo. Collectively, they identify SP140 as a TOP repressor and reveal repurposing of TOP inhibition to reverse immune diseases driven by SP140 loss.
2. Gut microbial DL-endopeptidase alleviates Crohn’s disease via the NOD2 pathway
The pattern-recognition receptor NOD2 senses bacterial muropeptides to regulate host immunity and maintain homeostasis. Loss-of-function mutations in NOD2 are associated with Crohn’s disease, but how the variations in microbial factors influence NOD2 signaling and host pathology is elusive. Hongwei Zhou, Microbiome Medicine Center, Department of Laboratory Medicine, Zhujiang Hospital, Southern Medical University, China, and his team demonstrated that the Firmicutes peptidoglycan remodeling enzyme, DL-endopeptidase, increased the NOD2 ligand level in the gut and impacted colitis outcomes[2]. Metagenomic analyses of global cohorts (n = 857) revealed that DL-endopeptidase gene abundance decreased globally in Crohn’s disease patients and negatively correlated with colitis. Fecal microbiota from Crohn’s disease patients with low DL-endopeptidase activity predisposed mice to colitis. Therapeutically restoring NOD2 ligands with a DL-endopeptidase-producing Lactobacillus salivarius strain or mifamurtide, a clinical analog of muramyl dipeptide, exerted potent anti-colitis effects (Fig.2). The study suggests that the depletion of DL-endopeptidase contributes to Crohn’s disease pathogenesis through NOD2 signaling, providing a therapeutically modifiable target.

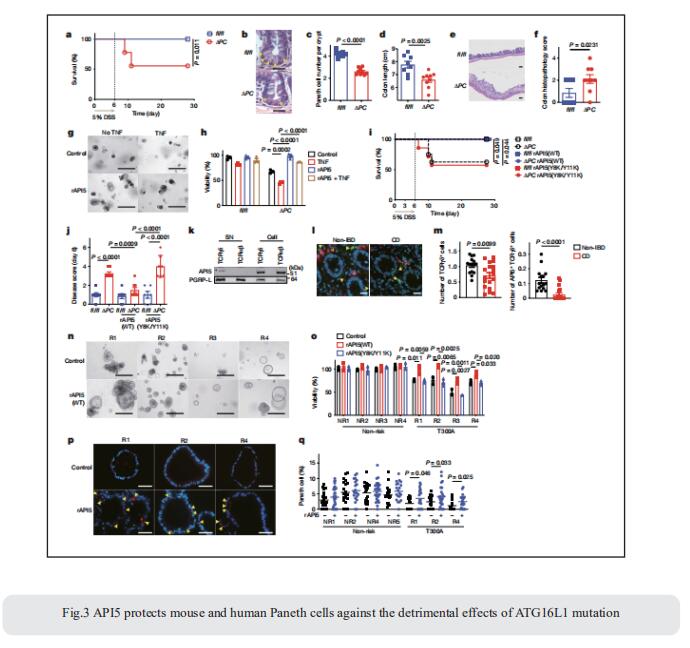
3. The γδ IEL effector API5 masks genetic susceptibility to Paneth cell death
Loss of Paneth cells and their antimicrobial granules compromises the intestinal epithelial barrier and is associated with Crohn’s disease. Intraepithelial lymphocytes (IELs), intercalate the intestinal epithelium. This anatomical position has implicated them as first-line defenders in resistance to infections, but their role in inflammatory disease pathogenesis requires clarification. The identification of mediators that coordinate crosstalk between specific IEL and epithelial subsets could provide insight into intestinal barrier mechanisms in health and disease. Ken Cadwell, Kimmel Center for Biology and Medicine at the Skirball Institute, New York University Grossman School of Medicine, USA, and his team showed that the subset of IELs that express γ and δ T cell receptor subunits (γδ IELs) promotes the viability of Paneth cells defcient in the Crohn’s disease susceptibility gene ATG16L1[3]. Using an ex vivo lymphocyte–epithelium co-culture system, they identifed apoptosis inhibitor 5 (API5) as a Paneth cell-protective factor secreted by γδ IELs. In the Atg16l1-mutant mouse model, viral infection induced a loss of Paneth cells and enhanced susceptibility to intestinal injury by inhibiting the secretion of API5 from γδ IELs. Therapeutic administration of recombinant API5 protected Paneth cells in vivo in mice and ex vivo in human organoids with the ATG16L1 risk allele (Fig.3). Thus, they identify API5 as a protective γδ IEL efector that masks genetic susceptibility to Paneth cell death.
References
[1]Amatullah H, Fraschilla I, Digumarthi S, et al. Epigenetic reader SP140 loss of function drives Crohn's disease due to uncontrolled macrophage topoisomerases [J]. Cell. 2022;185(17):3232-3247.e18. (IF=66.850)
[2]Gao J, Zhao X, Hu S, et al. Gut microbial DL-endopeptidase alleviates Crohn's disease via the NOD2 pathway [J]. Cell Host Microbe. 2022;30(10):1435-1449.e9. (IF=31.316)
[3]Matsuzawa-Ishimoto Y, Yao X, Koide A, et al. The γδ IEL effector API5 masks genetic susceptibility to Paneth cell death [J]. Nature. 2022;610(7932):547-554. (IF=69.504)
Cloud-Clone not only provides animal models of various digestive system diseases, including Crohn's disease, ulcerative colitis, irritable bowel syndrome, gastritis, duodenal ulcer and other common digestive disease animal models. We also have various digestive system diseases detection indicators and the above SP140, NOD2, API5 and other related products, which can help the vast number of researchers to carry out digestive system diseases related research.