New therapeutic targets for inflammatory bowel disease
Inflammatory bowel disease (IBD) is a chronic intestinal inflammatory disease that affects the ileum, rectum and colon. Crohn's disease (CD) and ulcerative colitis (UC) are two major forms of IBD. The global distribution of IBD tends to be more prominent in North America and Europe, but its prevalence has increased in developing countries such as China and India over the past 20 years.
Potential therapeutic targets for IBD
The etiology and pathogenesis of IBD have not been fully clarified. It is known that the inflammatory response caused by abnormal immune system reaction of intestinal mucosa plays an important role in the pathogenesis of IBD, which is believed to be caused by the interaction of multiple factors, mainly including environmental, genetic, infectious and immune factors. Recently, several articles have reported new insights into the pathogenesis of IBD, which may provide therapeutic targets for IBD.
1. Deficiency in X-linked inhibitor of apoptosis protein promotes susceptibility to microbial triggers of intestinal inflammation
Paneth cells are secretory intestinal epithelial cells, which regulate commensal intestinal microbial composition and limit the susceptibility to enteric pathogens through the secretion of antimicrobial peptides. Sebastian Zeissig at the Centre for Regenerative Therapy, Technische Universität Dresden, and his team demonstrated that the loss of X-linked inhibitor of apoptosis protein (XIAP), a gene associated with Mendelian IBD, rendered Paneth cells sensitive to microbiota-, tumor necrosis factor (TNF)–, receptor-interacting protein kinase 1 (RIPK1)–, and RIPK3-dependent cell death(Fig.1)[1]. This was associated with deficiency in Paneth cell–derived antimicrobial peptides and alterations in the stratification and composition of the microbiota. Loss of XIAP was not sufficient to elicit intestinal inflammation but provided susceptibility to pathobionts able to promote granulomatous ileitis, which could be prevented by administration of a Paneth cell–derived antimicrobial peptide. These data reveal a pathway critical for host-microbial cross-talk, which is required for intestinal homeostasis and the prevention of inflammation and which is amenable to therapeutic targeting.
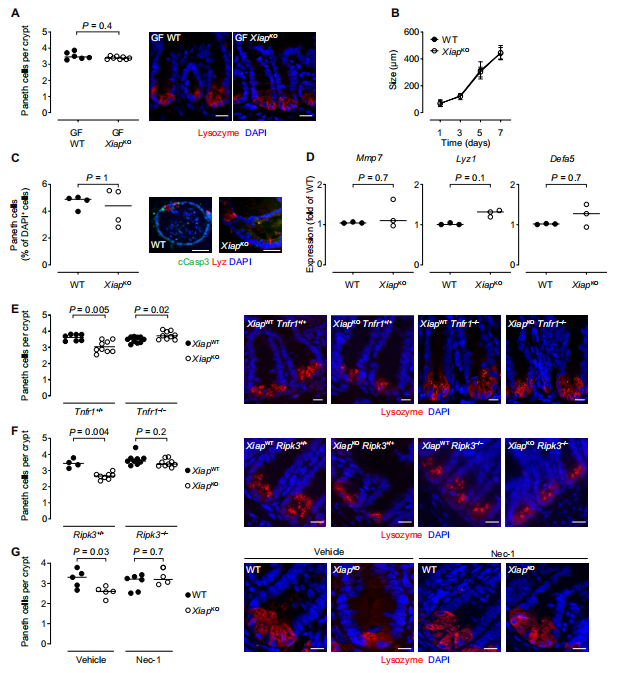
Fig.1 Paneth cell loss in XIAPKO mice is dependent on the microbiota, TNF, RIPK1, and RIPK3
2. Foxo3a tempers glutaminolysis to prevent fatal gut inflammation
Foxo3a has been found to play a role in regulating inflammation. Foxo3a signaling has been shown to negatively regulate the expression of IL-10. The impairment in IL-10 signaling has been shown to promote susceptibility to Crohn’s disease, and some strains of IL-10-deficient mice develop gut inflammation. Therefore, Subash Sad and his team investigated the effect of Foxo3a on intestinal inflammation in IL-10 deficient mice[2]. The reduction in Foxo3a expression in IL-10-deficient mice results in a spontaneous and aggressive IBD- like disease, which is rescued by deletion of myeloid cells, T cells and inhibition of mTORC1. Foxo3a Foxo3a signaling represses the transcription of glutaminase to prevent over-consumption of glutamine by activated T cells and its conversion to glutamate that contributes to the TCA cycle and mTORC1 activation(Fig.2). Thus, by suppressing glutaminolysis in activated T cells Foxo3a mediates a critical checkpoint that prevents the development of fulminant gut inflammatory disease.
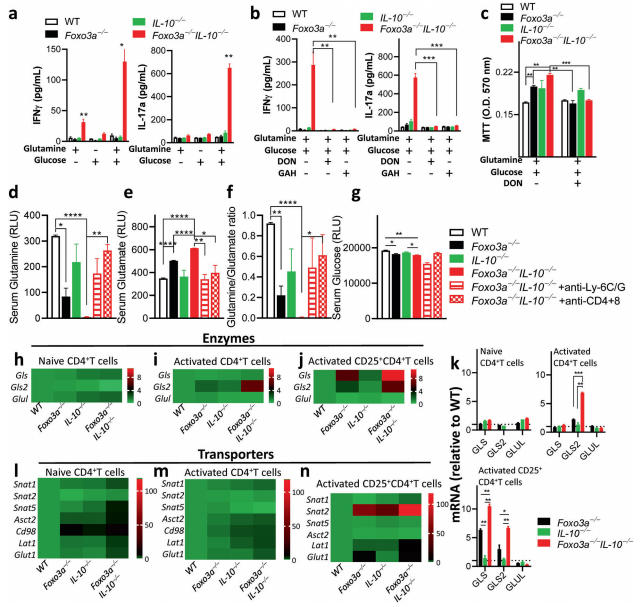
Fig.2 Foxo3a favors glutamine synthesis by repressing the expression of glutaminase and glutamine transporters
3. Serum metabolome analysis indicates new putative therapeutic targets for IBD
By analyzing serum metabolites from UC and CD patients, Carmen Argmann and her team at Icahn School of Medicine at Mount Sinai Carmen Argmann found that several metabolites were significantly associated with clinically defined IBD disease status and activity[3]. Network analysis implicated TMEM229B in IBD pathology, given that it is in close association with IL12RB1, IL10RA, SOCS1, TMEM137 and TTC7A, which are known to be involved in IBD pathogenesis(Fig.3). In addition, the reduction of plasmalogen was observed in TMEM229B overexpressed cells, supporting the reverse regulation of plasmalogen level by TMEM229B. Plasmalogen serves as reservoirs for lipid mediators and sacrificial oxidants to help terminate lipid oxidation, while IBD is associated with an increase in oxidative stress. Thus, the new association between serum plasmalogen and TMEM229B was predicted to be related to IBD. This knowledge provides potential understanding to IBD pathology as well as novel biomarkers and emphasizes the value of integrative multi-omic studies.
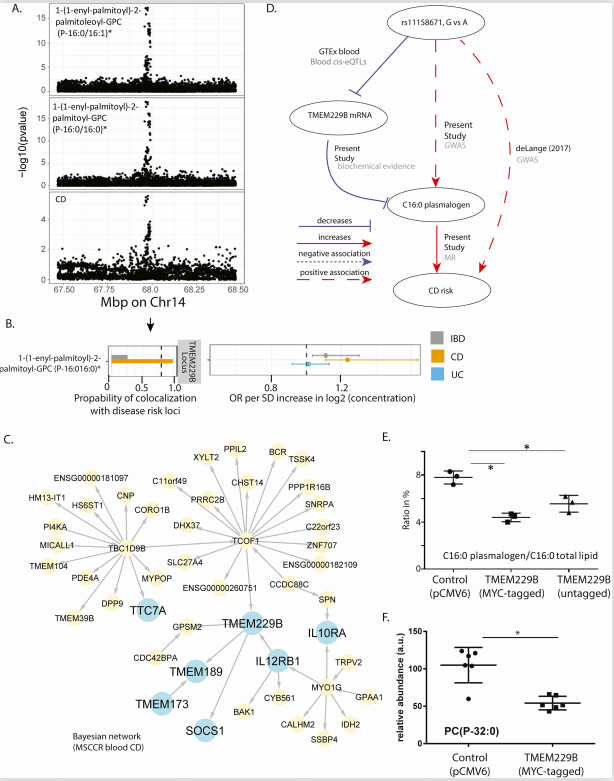
Fig.3 TMEM229B expression as candidate mediator of IBD risk
4. Protective Role of Spermidine in Colitis
Keith T. Wilson of Vanderbilt University Medical Center and his team previous studies have shown that spermidine oxidase (SMOX), which produces spermidine (Spd), regulates colitis. Recently, they determined if Spd treatment reduces colitis and carcinogenesis[4]. SMOX mRNA levels were decreased in UC tissues and inversely correlated with disease activity, and SMOX protein was reduced in colitis-associated dysplasia. DSS colitis and AOM-DSS-induced dysplasia and tumorigenesis were worsened in Smox–/– versus WT mice and improved in both genotypes with Spd(Fig.4). Smox deletion and AOM-DSS treatment were both strongly associated with increased expression of alpha-defensins, which was reduced by Spd. Those results indicated that Spd haspotential as an adjunctive treatment for colitis and chemopreventive for colon carcinogenesis.
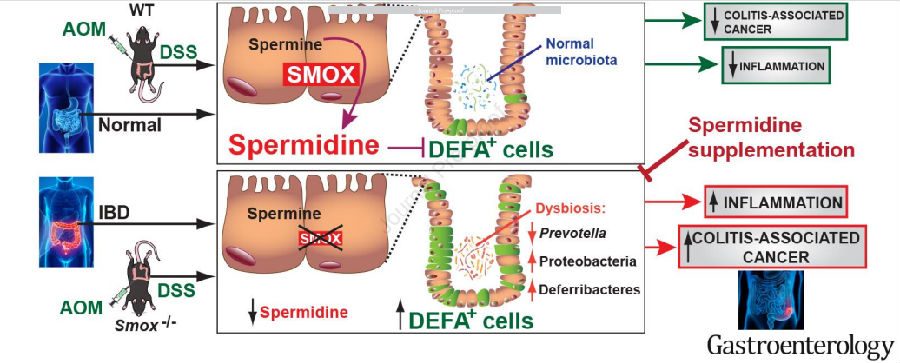
Fig.4 The disease of mouse model was improved after Spd supplement
References
[1]Strigli A, Gopalakrishnan S, Zeissig Y, et al. Deficiency in X-linked inhibitor of apoptosis protein promotes susceptibility to microbial triggers of intestinal inflammation [J]. Sci Immunol. 2021, 6(65):eabf7473. (IF=17.727)
[2]Hajjar S, Nathan N, Joseph J, et al. Foxo3a tempers excessive glutaminolysis in activated T cells to prevent fatal gut inflammation in the murine IL-10-/- model of colitis [J]. Cell Death Differ. 2021, 10.1038/s41418-021-00876-y. (IF=15.828)
[3]Di Narzo AF, Houten SM, Kosoy R, et al. Integrative analysis of the Inflammatory Bowel Disease serum metabolome improves our understanding of genetic etiology and points to novel putative therapeutic targets [J]. Gastroenterology. 2021, S0016-5085(21)03738-0. (IF=22.682)
[4]Gobert AP, Latour YL, Asim M, et al. Protective Role of Spermidine in Colitis and Colon Carcinogenesis [J]. Gastroenterology. 2021;S0016-5085(21)03727-6. (IF=22.682)
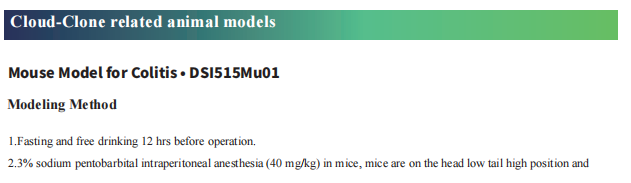
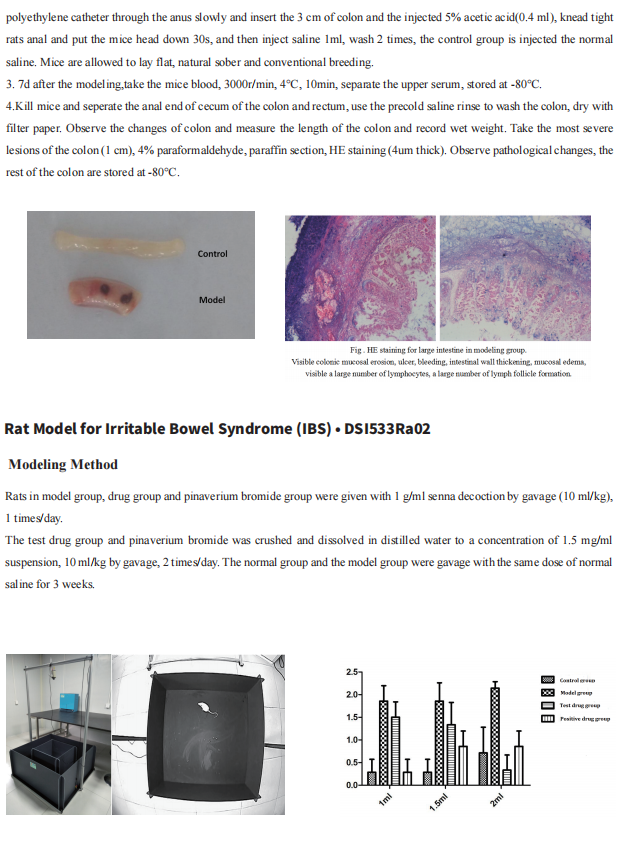
Cloud-Clone not only provides animal models of common digestive diseases such as colitis, irritable bowel syndrome, gastric ulcer, gastritis and duodenal ulcer, but also has products related to various gastrointestinal disease detection indicators, which can help the majority of scientific researchers to conduct researches on gastrointestinal diseases.