Plasmin activity promotes amyloid deposition in a transgenic model of human transthyretin amyloidosis

On December 7, 2021, J. Paul Simons, Division of Medicine, University College London, and his team published a paper tittled “Plasmin activity promotes amyloid deposition in a transgenic model of human transthyretin amyloidosis” in NATURE COMMUNICATIONS , which reported a mouse model of cardiac ATTR amyloidosis with human TTRS52P transgenic expression.
The kits [ELISA Kit for Thyroid Stimulating Hormone (TSH), CEA463Mu] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.


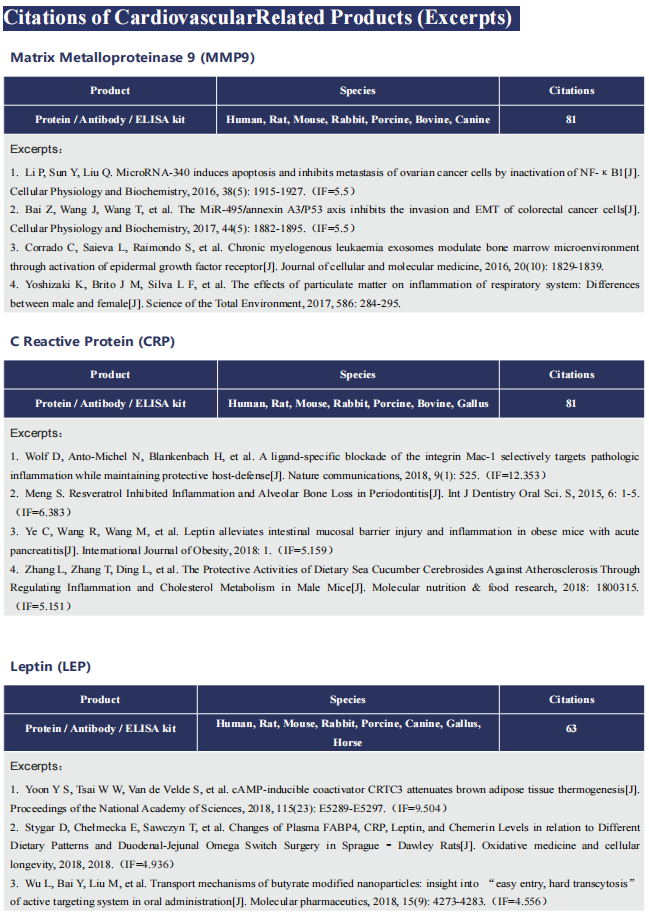
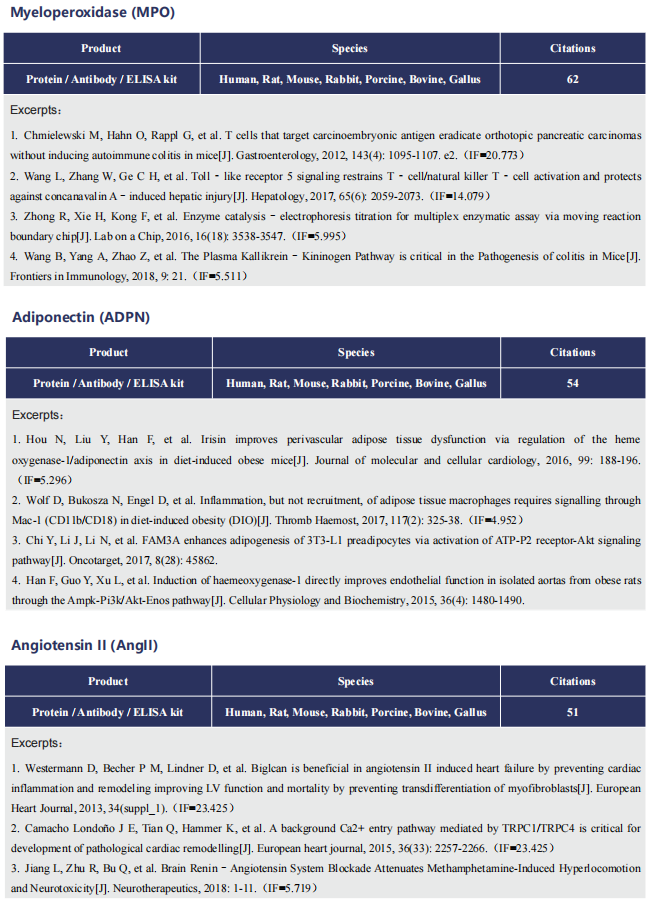
Cardiac ATTR amyloidosis, a serious but much under-diagnosed form of cardiomyopathy, is caused by deposition of amyloid fibrils derived from the plasma protein transthyretin (TTR), but its pathogenesis is poorly understood and informative in vivo models have proved elusive. Here we report the generation of a mouse model of cardiac ATTR amyloidosis with transgenic expression of human TTRS52P. The model is characterised by substantial ATTR amyloid deposits in the heart and tongue. The amyloid fibrils contain both full-length human TTR protomers and the residue 49-127 cleavage fragment which are present in ATTR amyloidosis patients. Urokinase-type plasminogen activator (uPA) and plasmin are abundant within the cardiac and lingual amyloid deposits, which contain marked serine protease activity; knockout of α2-antiplasmin, the physiological inhibitor of plasmin, enhances amyloid formation. Together, these findings indicate that cardiac ATTR amyloid deposition involves local uPAmediated generation of plasmin and cleavage of TTR, consistent with the previously described mechano-enzymatic hypothesis for cardiac ATTR amyloid formation. This experimental model of ATTR cardiomyopathy has potential to allow further investigations of the factors that influence human ATTR amyloid deposition and the development of new treatments.
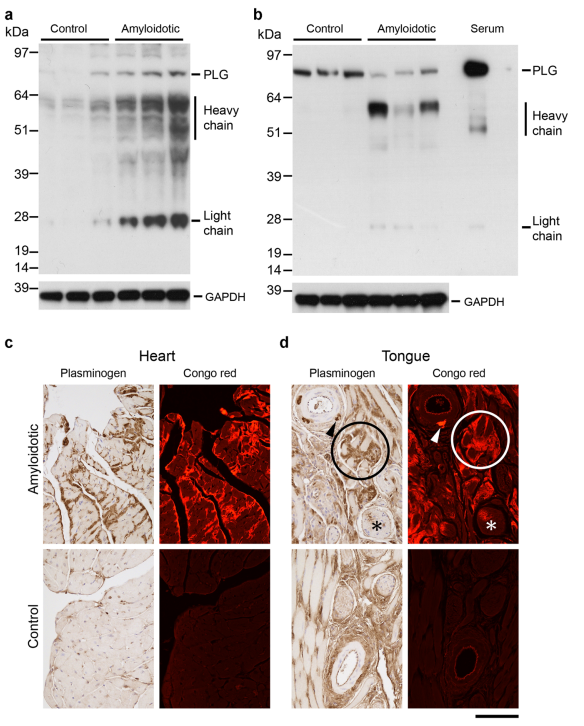
Fig. Association of plasmin(ogen) with amyloid deposits