Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging
Fertility and reproductive lifespan in humans decrease dramatically with age. Prospective female parents of advanced age may experience increased risk of infertility, spontaneous miscarriages, perinatal mortality and congenital anomaly. Although assisted reproductive technologies can compensate for human suboptimal reproductive outcome to some extent, the success rate of assisted reproductive technology cycles declines with advanced age as well. This tendency is mainly associated with age-related deterioration of ovarian reserve and oocyte quality, along with a higher aneuploidy rate.
On October 16, 2023, Bo Xiong, College of Animal Science and Technology, Nanjing Agricultural University, China, and his team published a paper titled “ Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging” in Nature Aging. Their findings suggest that spermidine supplementation could represent a therapeutic strategy to ameliorate oocyte quality and reproductive outcome in women trying to conceive at an advanced age.

The kit [ELISA Kit for Spermidine (SMD), CEX053Ge] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.

Spermidine is found in most cells and tissues, including the ovary. It participates in a wide range of cellular events, including regulation of transcription and translation, induction of oxidative stress, autophagy and apoptosis, because of its anti-inflammatory activities, antioxidative properties and reinforced mitochondrial functions. Although an increasing number of studies have been reported about the restorative role of spermidine for aging in somatic cells, the effect of spermidine on oocyte aging has not been clarified.
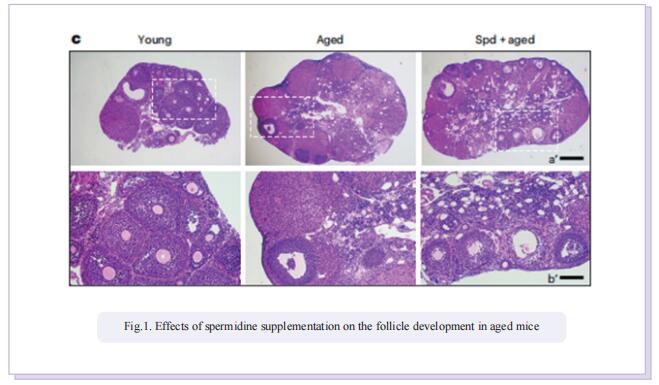
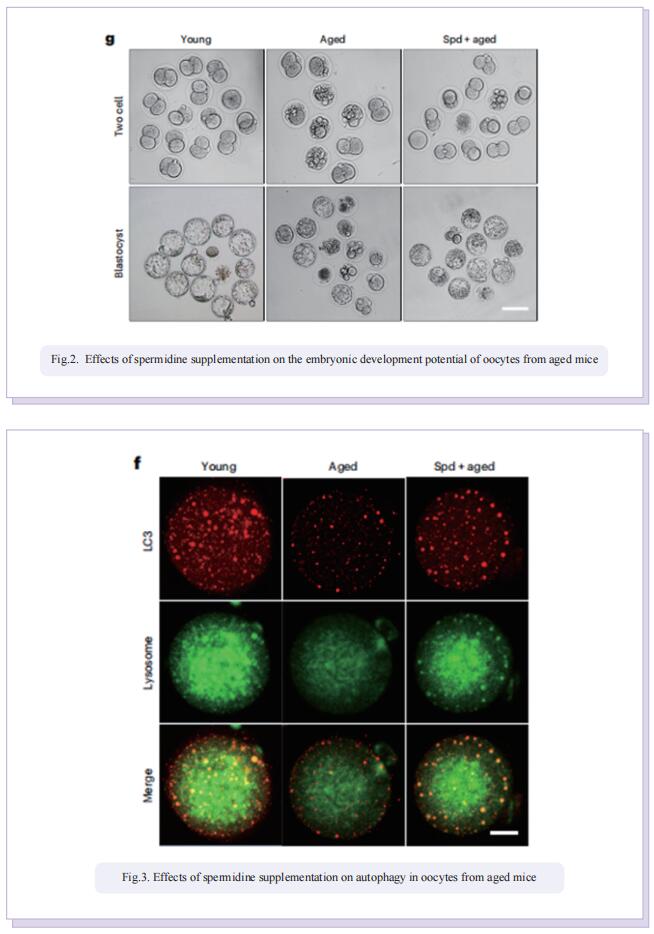
In this study, the authors applied untargeted metabolomics to identify spermidine as a critical metabolite in ovaries to protect oocytes against aging. In particular, they found that the spermidine level was reduced in ovaries of aged mice and that supplementation with spermidine promoted follicle development, oocyte maturation, early embryonic development and female fertility of aged mice. By microtranscriptomic analysis, they further discovered that spermidine-induced recovery of oocyte quality was mediated by enhancement of mitophagy activity and mitochondrial function in aged mice, and this mechanism of action was conserved in porcine oocytes under oxidative stress.
In sum, the study provides clinical implications for the potential application of spermidine to ameliorate the reproductive outcome of women at an advanced age by means of either natural pregnancy or assisted reproductive technology.