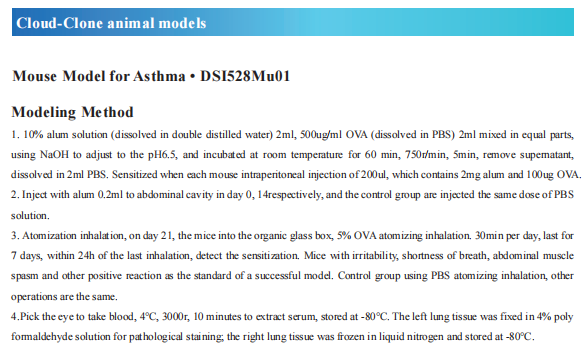
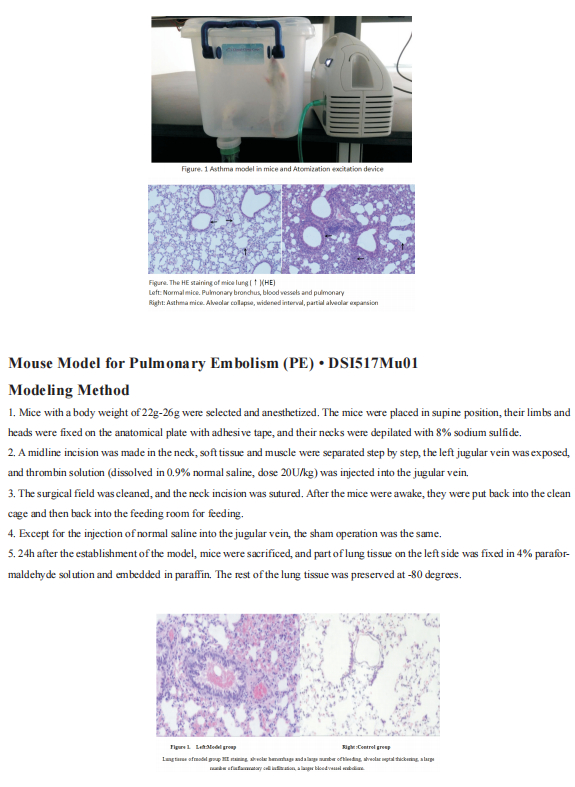
Recent advances in the pathogenesis of asthma
Asthma is a heterogeneous disease, characterized by airway hyperresponsiveness, airway inflammation and reversible airway remodeling. As a common chronic respiratory disease, asthma remains a serious health problem that affects 358 million individuals, especially among children. Asthma patients report respiratory symptoms such as dyspnoea, wheeze, cough and/or chest tightness. These symptoms increase in frequency and intensity in more severe disease, accompanied by expiratory airflow limitation and an overall decline in lung function.
Recent advances in the pathogenesis of asthma
The exact triggers and underlying molecular mechanisms causing acute exacerbations remain poorly defined, although viruses, bacteria, environmental toxins, ozone, and allergens are believed to contribute to acute exacerbations and progressive worsening of disease. Identifying triggers of disease exacerbation, the molecular consequences of exposure to such triggers, and points of therapeutic intervention to prevent hyperreactivity to such triggers could address a remaining unmet need for patients living with asthma.
1. Fine Particulate Matter Induces Childhood Asthma Attacks via Extracellular Vesicle-Packaged Let-7i-5p-Mediated Modulation of the MAPK Signaling Pathway
Fine particulate matter less than 2.5 μm in diameter (PM2.5) is a major risk factor for acute asthma attacks in children. Zhengdong Zhang, School of Public Health, Nanjing Medical University, and his team found that PM2.5-treated human bronchial epithelial (HBE) cells-secreted extracellular vesicles (PM2.5-EVs) caused cytotoxicity in “horizontal” HBE cells and increased the contractility of “longitudinal” sensitive human bronchial smooth muscle cells (HBSMCs)[1] . RNA sequencing showed that let-7i-5p is significantly overexpressed in PM2.5-EVs and asthmatic plasma; additionally, its level is correlated with PM2.5 exposure in children with asthma. Mechanistically, let-7i-5p is packaged into PM2.5-EVs by interacting with ELAVL1 and internalized by both “horizontal” recipient HBE cells and “longitudinal” recipient-sensitive HBSMCs, with subsequent activation of the MAPK signaling pathway via suppression of its target DUSP1 (Fig.1). The results not only revealed a crucial mechanism of EV-packaged miRNA-regulated cell–cell communication from PM2.5-treated HBE cells to recipient cells to induce asthma, but also opened a new avenue for a diagnostic strategy and therapeutic approach for asthma in children exposed to high levels of PM2.5.
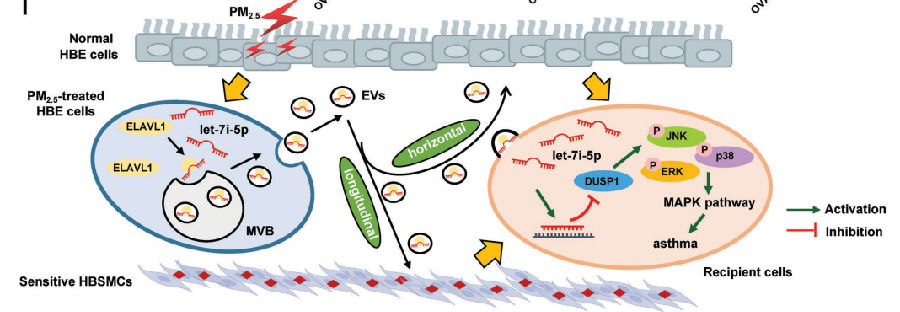
Fig.1 EV-packaged let-7i-5p secreted by PM2.5-treated HBE cells induce asthma by regulating the expression of its target gene DUSP1 and activating the MAPK signaling pathway in recipient cells
2. Involvement of autophagy in exacerbation of eosinophilic airway inflammation in a murine model of obese asthma
Obesity is a common comorbidity in patients with asthma, and obese asthma patients present the most refractory phenotype among patients with severe asthma. Takafumi Suda, Hamamatsu University School of Medicine, Japan, and his team hypothesized that autophagy is involved in the pathogenesis of obese asthma[2]. House dust mite (HDM)-sensitized atg5−/− obese mice exhibited marked eosinophilic inflammation and airway hyper-reactivity (AHR), compared to wild-type (WT) obese mice. Analyses of atg5−/− obese mice showed increased levels of Th2 cells but not ILC2s together with elevated expression of Th2 cytokines in the lung. HDM-sensitized atg5−/− mice developed TSLP- and IL33-dependent eosinophilic inflammation and AHR. The results suggest that autophagy contributes to the exacerbation of eosinophilic inflammation in obese asthma. Modulations of autophagy may be a therapeutic target in obesity-associated asthma.
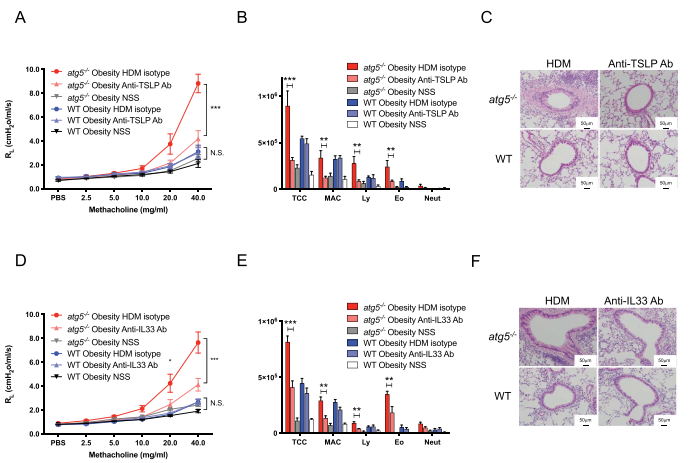
Fig.2 HDM-challenged atg5−/− obese mice develop TSLP-dependent and IL33-dependent asthma
3. Lysyl oxidase-like 2 is increased in asthma and contributes to asthmatic airway remodelling
Airway smooth muscle cells (ASM) are fundamental to asthma pathogenesis, influencing bronchoconstriction, airway hyper-responsiveness, and airway remodelling. Extracellular matrix (ECM) can influence tissue remodelling pathways. Amanda L Tatler, School of Medicine, University of Nottingham, UK, and his team hypothesised that TGFβ activation by ASM is influenced by ECM in asthma and sought to investigate the mechanisms involved[3]. They found that ASM cells from asthmatics activated more TGFβ basally than non-asthmatic controls and that diseased cell-derived ECM influences levels of TGFβ activated. The ECM crosslinking enzyme lysyl oxidase like-2 (LOXL2) is increased in asthmatic ASM cells and in bronchial biopsies. LOXL2 inhibition reduces ECM stiffness and TGFβ activation in vitro (Fig.3), and can reduce subepithelial collagen deposition and ASM thickness, two features of airway remodelling, in an ovalbumin mouse model of asthma. These data highlight a role for LOXL2 in the development of asthmatic airway remodelling and suggest that LOXL2 inhibition warrants further investigation as a potential therapy to reduce remodelling of the airways in severe asthma.
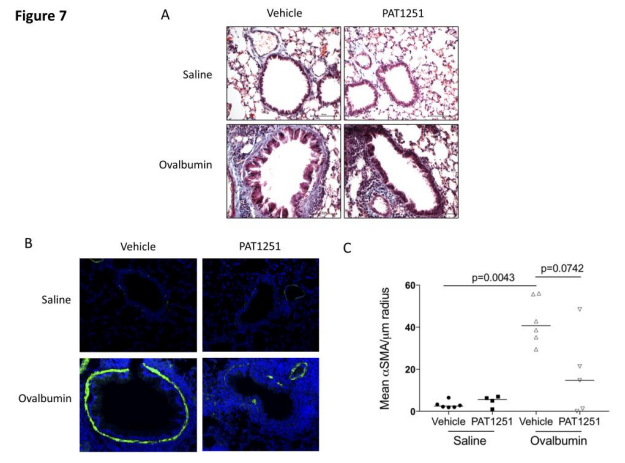
Fig.3 LOXL2 inhibition in vivo reduces chronic ovalbumin challenge-induced airway remodelling
4. Oncostatin M expression induced by bacterial triggers drives airway inflammatory and mucus secretion in severe asthma
Bacterial dysbiosis and opportunistic bacterial infections have been observed in, and may contribute to, more severe asthma. Mark S. Wilson, Genentech Inc., USA, and hia team showed that bacterial lipopolysaccharide (LPS) induces oncostatin M (OSM) and that airway biopsies from patients with severe asthma present with an OSM-driven transcriptional profile[4]. This profile correlates with activation of inflammatory and mucus-producing pathways. we demonstrate that OSM is necessary and sufficient to drive pathophysiological features observed in severe asthma after exposure to LPS or Klebsiella pneumoniae(Fig.4). These findings were further supported through blockade of OSM with an OSM-specific antibody. Using a model of Klebsiella pneumoniae, OSM blockade reduced airway neutrophilia and Muc5ac, Muc5b, and Clca1 gene expression without compromising expression of IL1a, IL1b, and IL6 or antibacterial immunity, which illustrates an important role of bacterial-driven OSM contributing to pathology rather than immunity. Together, these data provide rationale for inhibiting OSM to prevent bacterial-associated progression and exacerbation of severe asthma.
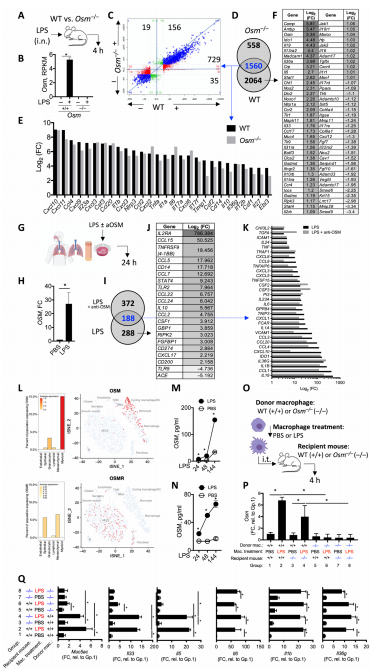
Fig.4 OSM drives tissue inflammation after exposure to LPS signals
References
[1] Zheng R, Du M, Tian M, et al. Fine Particulate Matter Induces Childhood Asthma Attacks via Extracellular Vesicle-Packaged Let-7i-5p-Mediated Modulation of the MAPK Signaling Pathway [J]. Adv Sci (Weinh). 2022, 9(3):e2102460.(IF=16.806)
[2] Suzuki Y, Aono Y, Akiyama N, et al. Involvement of autophagy in exacerbation of eosinophilic airway inflammation in a murine model of obese asthma [J]. Autophagy. 2022, 1-13.(IF=16.016)
[3] Ramis J, Middlewick R, Pappalardo F, et al. Lysyl oxidase-like 2 is increased in asthma and contributes to asthmatic airway remodelling [J]. Eur Respir J. 2022, 2004361.(IF=16.671)
[4] Headland SE, Dengler HS, Xu D, et al. Oncostatin M expression induced by bacterial triggers drives airway inflammatory and mucus secretion in severe asthma [J]. Sci Transl Med. 2022;14(627):eabf8188.(IF=17.956)
Cloud-Clone not only provides animal models of asthma, but also covers animal models of other common respiratory diseases (chronic obstructive pulmonary disease, bronchitis, pulmonary embolism, pneumonia, pulmonary fibrosis, etc.). It also has all kinds of lung disease detection index related products, which can help the majority of scientific researchers to carry out respiratory disease related research.