Simulated Growth of Virus
"Virus" literally means a disease-causing toxin, which coincides with its definition in modern medicine. Viruses are a unique class of infectious agents. They can use the nutrients of the host cells to autonomously replicate their own DNA, RNA, proteins and other life-constituent tiny living bodies. The distinctive feature of the virus is its simple structure, so small that it cannot even be observed with a common microscope, and it cannot be separated from the living body for independent metabolic activities and replication.
How to carry out corresponding scientific research on such a common but special virus?
It is not difficult to find from the characteristics of the virus, the key point for its survival is the living cells. With the in-depth study of various viruses, it is urgent to establish an in vitro infection model through susceptible cells. That is, the "pseudo" growth that mimics the survival and development of viruses mentioned in the title of this article.
We use Hepatitis B virus (HBV), which causes global public health problems, as the research object to conduct small-scale science experiments on in vitro infection simulation. HBV is a hepatotropic virus (Figure 1), and the liver is its main place of invasion and replication. Therefore, liver-derived cells become the first target cells for viral DNA transfection. The researchers chose differentiation after continuous subculture Liver cancer cells, such as the HepG2 cell line [1]。
Figure.1 Hepatitis B virus
There are two types of virus infection in vitro: one is to directly infect cells with virus (such as lymphocytes in bone marrow, human placental trophoblast cell line Bewo cells, etc. [2], observe the morphology of the virus, and determine whether the cells can be infected by the virus in vitro Infection and the study of the interaction between viruses and cells provide a basis for the subsequent establishment of virus detection, infection prevention and treatment.
In order to study the function of viral genome in a deeper level, we elaborate the mechanism of hepatitis B virus embedded in human genome and part of the mechanism of viral carcinogenesis. By constructing a recombinant adenovirus vector of hepatitis B virus gene, the recombinant adenovirus carrying the HBV gene was used to infect HEK293 cells for packaging, expansion, isolation and purification, and the amplified HBV was infected to HepG2 cells [3]. In view of the establishment of the virus infection cell model in vitro is helpful for the basic research of virus particle morphology, pathogenic mechanism, replication and expression, and the progress of the basic research also promotes the establishment of the cell infection model in vitro [4-5].
Based on the success of the cell model, more researchers have set their sights on animal models. The team of Professor Xia Ning Shao of Xiamen University and the team of Professor Li Jun of the First Affiliated Hospital of Zhejiang University have worked together for 5 years to establish the world ’s first mouse model of chronic hepatitis B cirrhosis induced by the natural infection of human HBV model. This is the first time that a single human BMSC transplantation at home and abroad has achieved the dual chimerism of human homologous hepatocytes and multiple immune cells in mice. It successfully simulated the natural infection of human HBV and the pathogenesis of hepatitis B cirrhosis, solving Infection and pathogenesis research and the development of new drugs against hepatitis B cirrhosis lack the international problems of high-quality animal models.
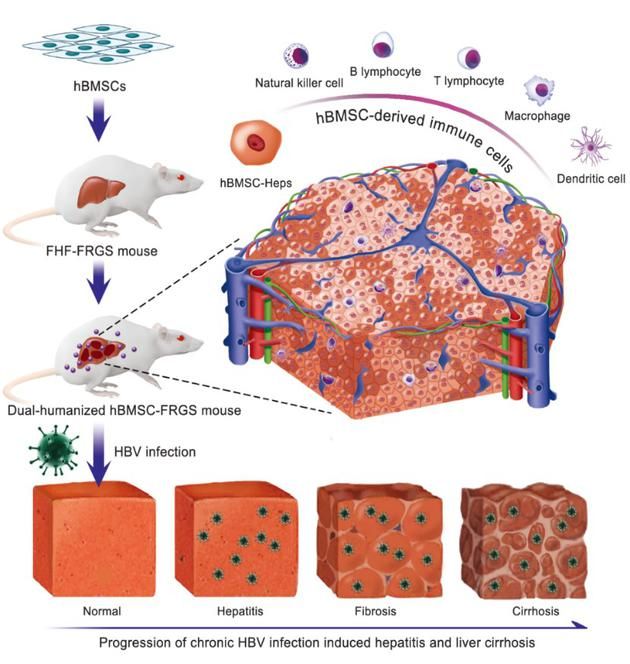
Figure 2. Mouse model of chronic hepatitis B cirrhosis induced by natural infection of human hepatitis B virus (HBV)
Cloud-Clone has been committed to serving global scientific researchers, providing high-quality testing products and experimental services. We are well aware of the importance of cell infection models. Our company can currently provide mature adenovirus and lentiviral packaging experiment services, and can also make more than 1,000 animal models, involving various human disease animal models, transgenic animal models and gene knockout Animal model. Designed to escort your experiment.
[Service Item Content]
I. Viral packaging experiment service
Content | Process | Period |
Experimental confirmation | 1. Customer information analysis 2. Determine experiment plan | 1w |
Acquirement of target gene | 1. PCR to obtain the target gene fragment 2. Genetic chemical synthesis | 2-4w |
Construction of vector | 1. Construction of vector 2. Plasmid extraction and endotoxin removal treatment | 2-3w |
Co-transfection | 1. Co-transfection of expression plasmid and helper plasmid into 293 (adenovirus) / 293T cells (lentivirus) 2. Cell culture | 1w |
Recombinant virus collection | 1. Recombinant virus collection, concentration, packaging and storage 2. Recombinant virus titer detection | 1w |
II. Tumor transplantation (TT) mouse model (example)
At present, in the study of liver cancer, tumor allograft injection method is used to inject tumor cell lines under the skin of the animal to form a local tumor mass to build a transplanted tumor model. Compared with the formation of primary liver cancer and pathophysiological changes in the body, there are still certain differences. By injecting liver cancer cells into the liver of nude mice to establish an in situ tumor model of the liver and observing the in situ tumor formation process and status, it can better simulate the growth process of human hepatocellular carcinoma and provide an experimental animal model for further anti-hepatoma immunity research.
1. Cell line: Human liver cancer cell line HepG2-luc labeled with luciferase gene.
2. Establishment of an orthotopic tumor model of nude mice liver:
2.1 Digest and centrifuge the HepG2-luc cells cultured under standard conditions in the logarithmic growth phase, and dissolve the resulting approximately 5 × 10 6 cells in 0.1 mL DMEM culture solution for use.
2.2 Anesthetize mice with 3% pentobarbital sodium. After anesthesia takes effect, open the liver and expose the liver. Use a 1ml insulin injection needle to take the prepared cells into the liver of nude mice. Suture the wound with 5-0 silk thread. To disinfect the wound, place the nude mouse on a heating pad and feed it in a normal diet after waking up.
3. Fluorescence in vivo imaging detection: Bioluminescence in vivo imaging detection is performed on nude mice after tumor formation. Mice are anesthetized with 3% pentobarbital sodium before imaging, and then 3 mg of substrate luciferin is injected intraperitoneally, 20 ~ after substrate injection After 30 minutes, bioluminescence in vivo imaging detection was performed.
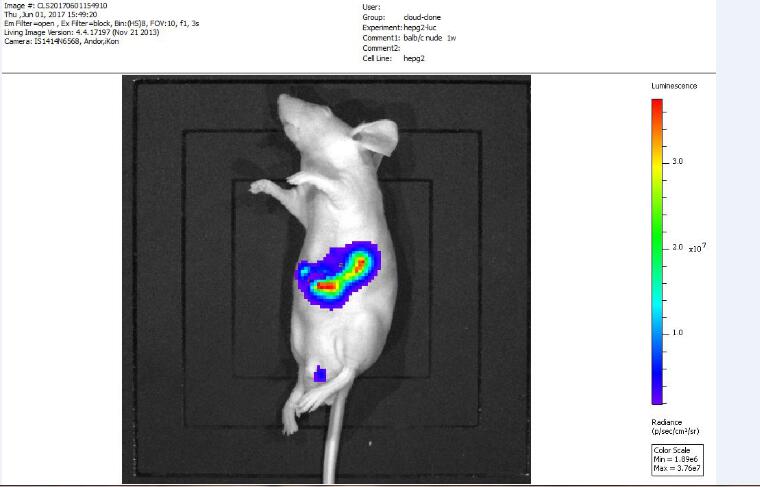
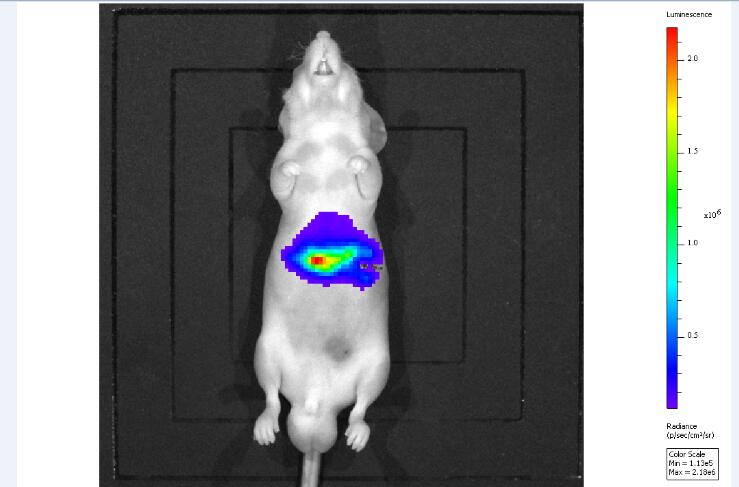
Figure 3. Bioluminescence in vivo imaging test results
Reference:
[1] Abraham T M , Lewellyn EB, Haines K M,et al.Characterization Of the contribution of spliced RNAs of hepatitis B virus to DNA synthesis intransfected cultures of Huh7 and HepG2 cells[J].Virology,2008,379:30-7.
[2] 刘明慧. 乙型肝炎病毒感染Bewo细胞模型的建立[J]. 中国妇幼保健, v.26(31):4901-4903.
[3] Benjamin Y. Winer et al, Analysis of host responses to hepatitis B and delta viral infections in a micro-scalable hepatic co-culture system, Hepatology (2019). DOI: 10.1002/hep.30815.
[4] 刁志宏,张明霞,朱幼芙,等.重组乙型肝炎病毒P22e基因在HepG2细胞中的表达[J].第四军医大学学报,2006,27(11):1008-10.
[5] 王安辉 徐德忠 门可 闫水平 张景霞 卢娟.乙型肝炎病毒体外感染HepG2细胞的初步研究[J].世界感染杂志,2005,(06):536-536页.
