Targeting insulin resistance to treat metabolic diseases
Metabolic diseases, including diabetes, obesity, non-alcoholic fatty liver disease (NAFLD), hyperlipidemia, and gout, have become major public health issues worldwide. Current therapy for metabolic diseases includes lifestyle interventions, balanced diet, appropriate exercise, and the use of pharmacological drugs. However, these drugs still have some limitations because of adverse effects.
Targeting insulin resistance to treat metabolic diseases
Insulin resistance (IR) is a common pathophysiological condition in which patients present with reduced insulin sensitivity and thus glucose intolerance, and unable to obtain to process the required energy from glucose to maintain cellular metabolic processes. IR is of major global concern as it is well-established as underpinning many chronic health conditions including type 2 diabetes mellitus (T2DM), obesity, cardiovascular disease polycystic ovary syndrome (PCOS), NAFLD, hypertension, and stroke. Moreover, understanding the molecular mechanisms that lead to IR will help facilitate the development of novel therapeutic strategies to prevent or lessen disease progression.
1. Macrophage deletion of NOC4L triggers endosomal TLR4/TRIF signal and leads to IR
In obesity, there is an increased accumulation of proinflammatory macrophages in insulin target tissues, including adipose, muscle, and liver, thus driving low-grade chronic systemic inflammation (LSI). The increased production of proinflammatory cytokines, such as TNFα and IL-6, was linked to IR via serine phosphorylation of IRS-1. Xiangdong Li. College of Biological Sciences, China Agricultural University, and his team found that NOC4L was decreased in both obese human and mice[1]. The macrophage-specific deletion of Noc4l in mice displayed IR and LSI. Conversely, Noc4l overexpression by lentivirus treatment and transgenic mouse model improved glucose metabolism in mice. NOC4L was localized in the endosome of Bone marrow derived macrophages and played a potential role in TRIF-dependent pathway by directly interacting with TLR4. It might inhibit the endocytosis of TLR4, thus reducing the production of IFNβ and proinflammatory cytokines, ameliorating the LSI and IR(Fig.1).
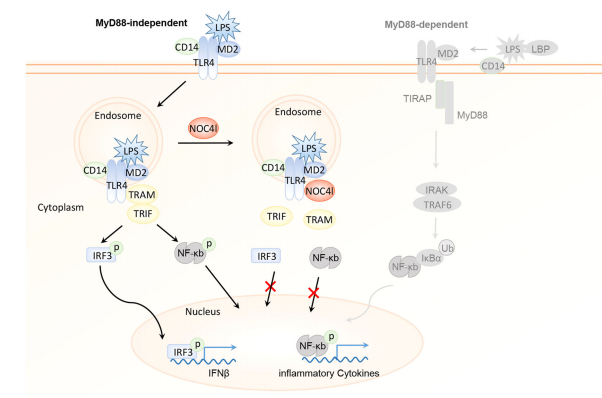
Fig.1 NOC4L blocks endocytosis of TLR4, thus inhibiting TRIF signal transduction
2. Targeting p21Cip1 highly expressing cells in adipose tissue alleviates IR
Senescent cells accumulate in multiple tissues with obesity and aging, and they secrete a variety of pro-inflammatory cytokines, chemokines, and proteases, termed the senescence-associated secretory phenotype (SASP). A role for cellular senescence in promoting metabolic dysfunction has been recently identified. Ming Xu of UConn Health and his team using single-cell transcriptomics, identified p21Cip1 highly expressing (p21high) cells, which accumulate in adipose tissue with obesity[2]. By leveraging a p21-Cre mouse model, they demonstrated that intermittent clearance of p21high cells can both prevent and alleviate IR in obese mice. Exclusive inactivation of the NF-kB pathway within p21high cells, without killing them, attenuates IR. Moreover, fat transplantation experiments establish that p21high cells within fat are sufficient to cause IR in vivo. Dasatinib plus quercetin, eliminates p21high cells in human fat ex vivo and mitigates IR following xenotransplantation into immuno-deficient mice(Fig.2). These findings lay the foundation for pursuing the targeting of p21high cells as a new therapy to alleviate IR.
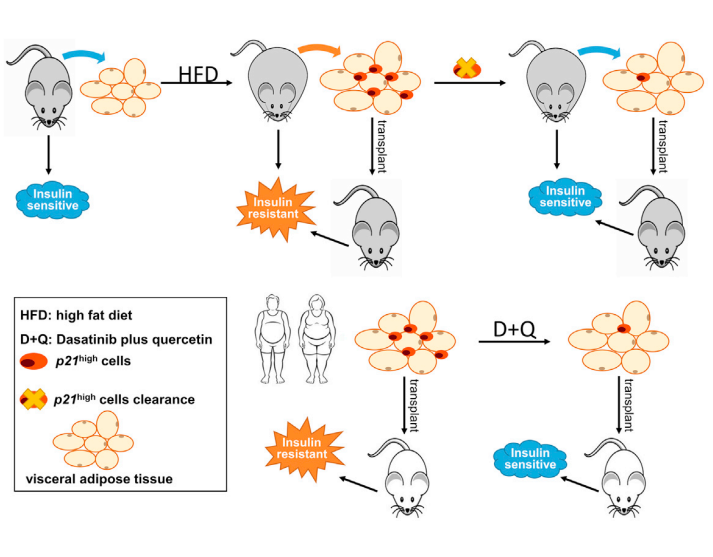
Fig.2 Targeting p21Cip1 highly expressing cells in adipose tissue alleviates IR
3. Natural killer cell-derived exosomal miR-1249-3p attenuates IR and inflammation in mouse models of T2DM
Immune system regulates metabolic organs throughout the body, and NK cells and macrophages can cooperate to regulate inflammation. Hanmei Xu, China Pharmaceutical University, and his team showed that NK-derived exosomes from lean mice attenuate obesity-induced IR and inflammation in mice of T2DM[3]. MiR-1249-3p, which is significantly upregulated in lean NK-derived exosomes, can be transferred from NK cells to adipocytes and hepatocytes via exosomes. NK-derived exosomal miR-1249-3p dramatically induces cellular insulin sensitivity and relieves inflammation. Mechanistically, exosomal miR-1249-3p directly targets SKOR1 to regulate the formation of ternary complex SMAD6/MYD88/SMURF1, which mediates glucose homeostasis by suppressing the TLR4/NF-κB signaling pathway(Fig.3). This study reveals an emerging role for NK-derived exosomal miR-1249-3p in remission of IR, and provides a series of potential therapeutic targets in T2DM.
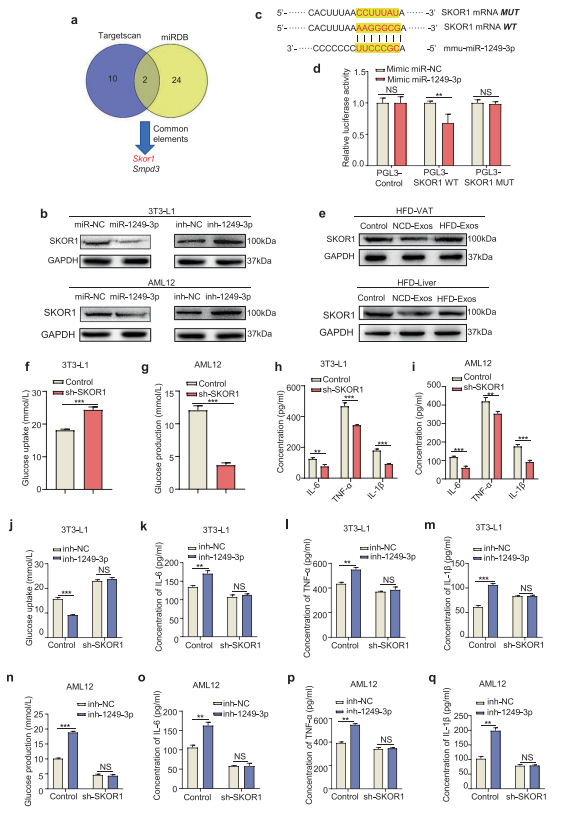
Fig.3 Exosomal miR-1249-3p directly targets SKOR1 to mediate insulin sensitivity
References
[1]Qin Y, Jia L, Liu H, et al. Macrophage deletion of Noc4l triggers endosomal TLR4/TRIF signal and leads to insulin resistance[J]. Nat Commun. 2021, 12(1):6121. (IF=14.919)
[2]Wang L, Wang B, Gasek NS, et al. Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity [J]. Cell Metab. 2021, S1550-4131(21)00530-1.(IF=27.287)
[3]Wang Y, Li M, Chen L, et al. Natural killer cell-derived exosomal miR-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes[J]. Signal Transduct Target Ther. 2021, 6(1):409. (IF=18.187)
Cloud-Clone can not only provide animal models of various metabolic diseases, including diabetes, high uric acid, non-alcoholic fatty liver and other common diseases. It also has various metabolic diseases detection indicators and the TLR4 and NF-KB signal pathway related products, which can help the general scientific research workers to carry out related research on metabolic diseases.