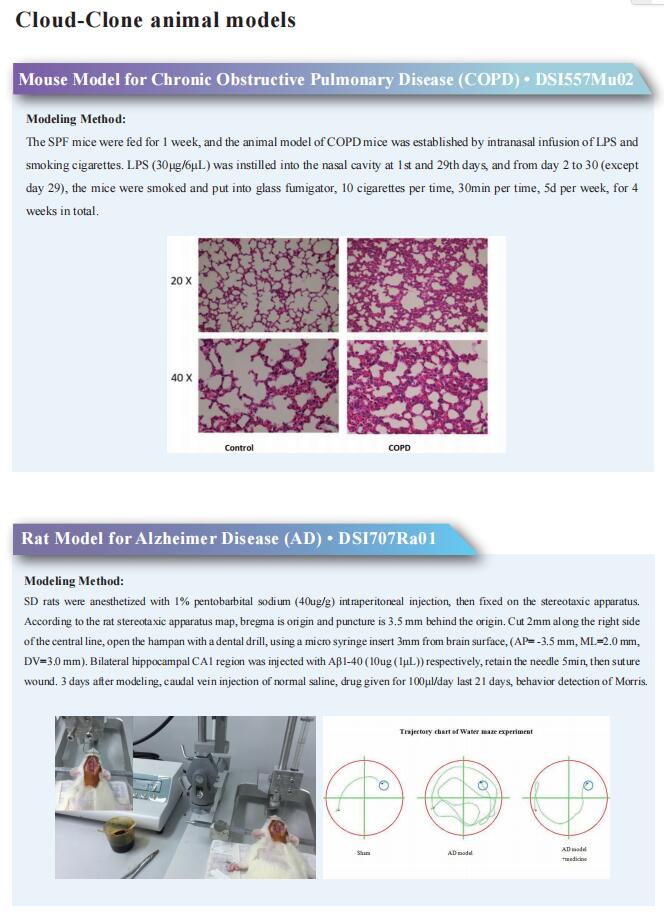
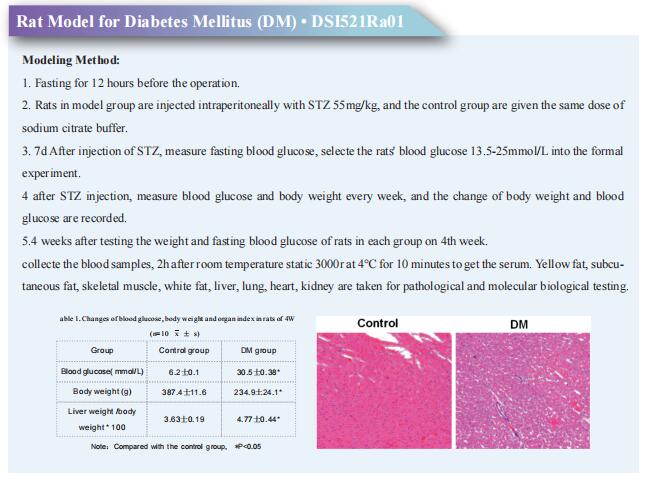
p16High senescence restricts cellular plasticity during somatic cell reprogramming
Research into rejuvenation and life extension with the aim of developing relevant therapies is undergoing a renaissance. One such approach has been to leverage the four Yamanaka reprogramming factors to counteract the in vivo signs of aging and increase the lifespan of mice with a premature-aging disease. The concern, however, is that epigenetic reprogramming and its impact on rejuvenation could be notably attenuated or even lost in fully aged tissues owing to profound changes in the epigenome of old cells, persistent chronic inflammation, profound tissue fibrosis and other age-related debilitating conditions. Senescent cells as one of the key drivers of aging-induced tissue deterioration, whereas removal of some senescent cells showed great promise in ameliorating several age-related conditions and extending the lifespan of mice. Senescent cells were found during early embryonic development, and initial observations revealed their negative role in somatic cell reprogramming in vitro. However, how reprogramming relates to aging remains to be studied.
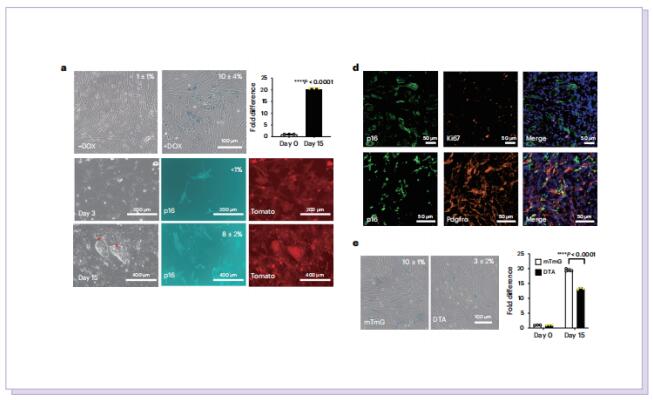
On August 31, 2023, Dmitry V. Bulavin, Institute for Research on Cancer and Aging of Nice (IRCAN), Université Côte d’Azur, INSERM, CNRS, Nice, France, and his team published a paper titled “p16High senescence restricts cellular plasticity during somatic cell reprogramming” in Nature Cell Biology. The study showed that the presence of p16High senescent cells limits cell plasticity, whereas their depletion can promote a totipotent-like state and histopathological tissue rejuvenation during reprogramming.

The antibody [Polyclonal Antibody to S-Adenosyl Methionine (SAM), PAG414Ge01] of Cloud-Clone brand was chosed in this article, we are so proud for supporting the reaserchers.

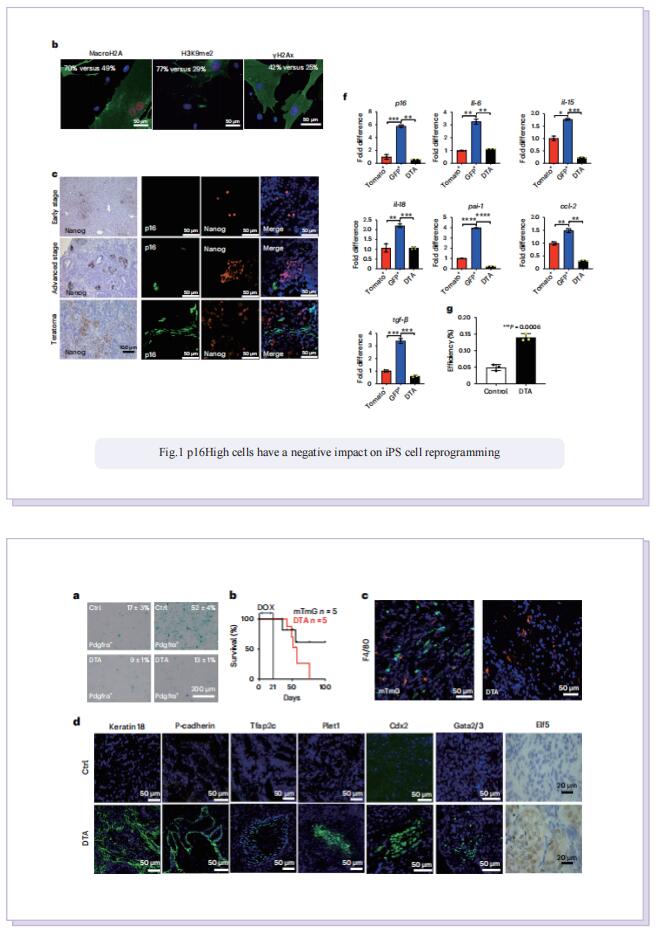
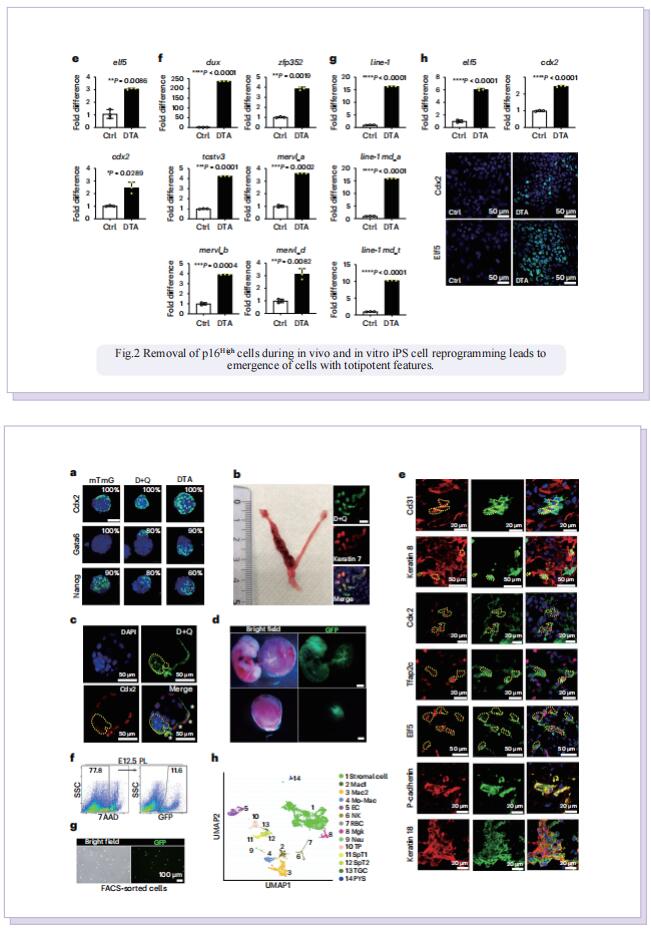
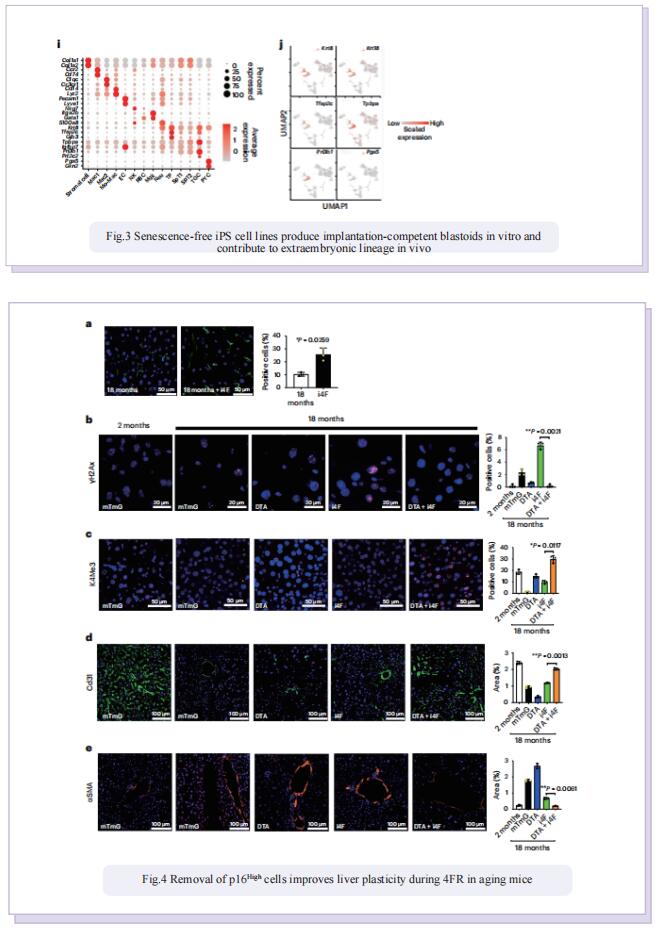
In this study, to dissect the role of senescence in iPS cell reprogramming, the authors used genetic and chemical approaches to remove p16High cells. They found that the removal of p16High senescent cells allowed achieving a high state of methylation-dependent epigenetic remodelling to support greater cellular plasticity. As a result, the iPS cell lines that emerged on senescence-deficient backgrounds expressed numerous markers of 2CLCs with features of experimental totipotency, readily formed implantation-competent blastoids and, following morula aggregation, contributed to embryonic and extraembryonic lineages. The effect of p16High senescent cells on iPS cell reprogramming appeared to be linked with negative regulation of the level of S-adenosyl-l-methionine (SAM). Senescence-dependent regulation of nicotinamide N-methyltransferase as a key mechanism controlling the SAM levels during four-factor (4F)-induced reprogramming (4FR) that was required for expression of the two-cell genes and acquisition of an extraembryonic potential. They further extended to rejuvenation induced by 4FR in vivo. 4FR, although inducing epigenetic rejuvenation, on its own failed to reverse aging-induced histopathological changes in the liver. By combining 4FR with the removal of p16High cells, significantly rejuvenating the vascular endothelial network and reducing liver fibrosis in aged mice.
Altogether, these findings indicate that removal of p16High senescent cells not only quantitatively and qualitatively enhances reprogramming of somatic cells into a totipotent-like state but also enables the reprogramming factors to induce an organ-level reversal of several markers of aging.