Packages (Simulation)

Reagent Preparation
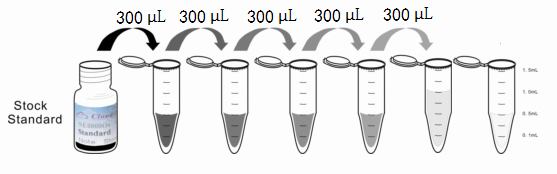
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay)
iPTH; Intact Parathyroid Hormone; Parathormone; Parathyrin
(Note: Up to 8-plex in one testing reaction)
- Product No.LMA866Ra
- Organism SpeciesRattus norvegicus (Rat) Same name, Different species.
- Sample Typeserum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodCompetitive Inhibition
- Assay Length1.5h
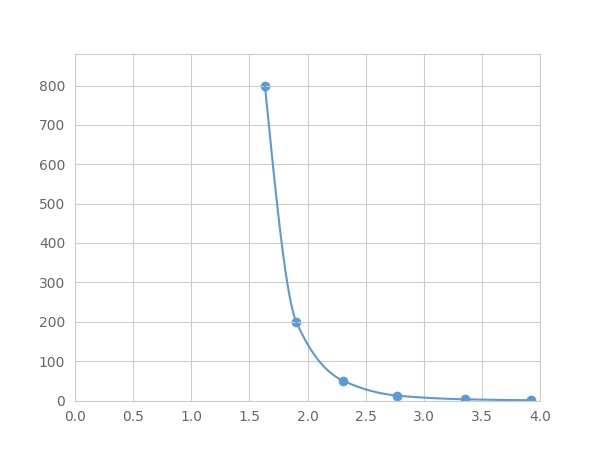
- Detection Range0.78-800pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.26 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 427
US$ 443
US$ 468
US$ 501
US$ 534
US$ 583
US$ 657
US$ 821
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 92-104 | 99 |
| EDTA plasma(n=5) | 80-92 | 86 |
| heparin plasma(n=5) | 79-92 | 78 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 82-91% | 79-91% | 87-101% | 97-105% |
| EDTA plasma(n=5) | 78-101% | 78-89% | 97-105% | 98-105% |
| heparin plasma(n=5) | 92-101% | 93-101% | 82-101% | 87-95% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:PTH) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 50μL standard or sample to each well,
add 10μL magnetic beads,and 50μL Detection Reagent A,incubate 60min at 37°C on shaker;
3. Wash plate on magnetic frame for three times;
4. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
5. Wash plate on magnetic frame for three times;
6. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Biological trace element research | Elevation of PTH and PTHrp Induced by Excessive Fluoride in Rats on a Calcium-deficient Diet PubMed: 19915804 |
| Basic nutritional investigation | Effects of duodenal redox status on calcium absorption and related genes expression in high-fat diet–fed mice ScienceDirect: S0899900709004808 |
| The Korean Journal of Nutrition | Effects of a Low Calcium Diet and Oxalate Intake on Calcium Deposits in Soft Tissues and Bone Metabolism in Ovariectomized Rats KoreaMed: source |
| Acta Physiologica Hungarica | Whole body vibration is a safe exercise training method and induces no impaired alterations on rat plasma parameters Pubmed: 22173025 |
| Toxicology and Industrial Health | The effect of supplementation of calcium, vitamin D, boron, and increased fluoride intake on bone mechanical properties and metabolic hormones in rat PubMed: 22782709 |
| Indian Journal of Experimental Biology | Effect of consumption of fatty acids, calcium, vitamin D and boron with regular physical activity on bone mechanical properties and corresponding metabolic hormones in rats. PubMed: 22439438 |
| Plos one | Is Gastrectomy-Induced High Turnover of Bone with Hyperosteoidosis and Increase of Mineralization a Typical Osteomalacia? PubMed: PMC3679169 |
| Nutr Hosp. | Effect of the “protein diet” and bone tissue. Pubmed: 24483972 |
| Clinical Cancer Research | Zoledronic acid has differential anti-tumour activity in the pre-and post-menopausal bone microenvironment in vivo Aacrjournals: Source |
| Nutricion Hospitalaria | Effect of the “protein diet” and bone tissue Aulamedica: Source |
| Academic Journal | Effect of whole body vibration on healthy rat plasma parameters Ebscohost: Source |
| J Proteome Res. | Biomarkers identified by urinary metabonomics for noninvasive diagnosis of nutritional rickets Pubmed:25051233 |
| Medicine (Baltimore) | An Attempt to Evaluate Selected Aspects of “Bone–Fat Axis” Function in Healthy Individuals and Patients With Pancreatic Cancer PubMed: 26266370 |
| J Nutr Sci Vitaminol (Tokyo) | The Impact of Different Amounts of Calcium Intake on Bone Mass and Arterial Calcification in Ovariectomized Rats PubMed: 26639847 |
| The Korean Society of Pharmacognosy | Effects of Oryza sativa L. Aleurone Layer Extract on Bone Mineral Density and Bone-related Markers in the Ovariectomized Rat Article: Hksobf_2015_V46N2_167 |
| J Bone Miner Res | Fat and Sucrose Intake Induces Obesity‐Related Bone Metabolism Disturbances: Kinetic and Reversibility Studies in Growing and Adult Rats Pubmed:26175082 |
| 7 | Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. pubmed:28218908 |
| PLOS ONE | Prostaglandin-E2 Mediated Increase in Calcium and Phosphate Excretion in a Mouse Model ofDistal Nephron Salt Wasting. pubmed:27442254 |
| International Journal of Advanced Research in Biological Sciences | Effects of fatty acids, nutrients and whole body vibration on bone histomorphometry, mechanical properties and metabolic parameters in male rat 10.22192/ijarbs.2017.04.04.018 |
| American Journal of Physiology-Renal Physiology | Chronic nicotine exposure reduces klotho expression and triggers different renal and hemodynamic responses in -haploinsufficient mice Pubmed:29363324 |
| Tropical Animal Health and Production | Dietary supplementation for Santa Inês hair ewes on pasture at pre-and postpartum periods: dry matter intake, digestibility, milk production, and mineral metabolism Pubmed:29931604 |
| International Immunopharmacology | CD8+ T lymphocytes enhance the anabolic effect of intermittent parathyroid hormone on cementoblasts Pubmed: 31679847 |
| ENDOCRINOLOGY | A New Therapeutic Approach Using a Calcilytic (AXT914) for Post-Surgical Hypoparathyroidism in Female Rats Pubmed: 32852547 |
| Kidney International | Calciprotein particles regulate fibroblast growth factor-23 expression in osteoblasts. |
| PREVENTIVE AND/OR THERAPEUTIC AGENT FOR OSTEOGENESIS IMPERFECTA AND OTHER DISEASES | |
| Oxid Med Cell Longev | Long-Term Administration of Abacavir and Etravirine Impairs Semen Quality and Alters Redox System and Bone Metabolism in Growing Male Wistar Rats 34373766 |
| Gene Therapy | Correction of a knock-in mouse model of acrodysostosis with gene therapy using a rAAV9-CAG-human PRKAR1A vector 34599290 |
| Cells | Calreticulin Shortage Results in Disturbance of Calcium Storage, Mitochondrial Disease, and Kidney Injury Pubmed:35456008 |
| African Health Sciences | Fibroblast Growth Factor 23 (FGF 23) and intact parathyroid hormone (iPTH) as markers of mineral bone disease among Nigerians with non-diabetic kidney disease |
| Catalog No. | Related products for research use of Rattus norvegicus (Rat) Organism species | Applications (RESEARCH USE ONLY!) |
| APA866Ra01 | Active Parathyroid Hormone (PTH) | Cell culture; Activity Assays. |
| RPA866Ra01 | Recombinant Parathyroid Hormone (PTH) | Positive Control; Immunogen; SDS-PAGE; WB. |
| CPA866Ra11 | BSA Conjugated Parathyroid Hormone (PTH) | Immunogen; SDS-PAGE; WB. |
| CPA866Ra21 | OVA Conjugated Parathyroid Hormone (PTH) | Immunogen; SDS-PAGE; WB. |
| PAA866Ra08 | Polyclonal Antibody to Parathyroid Hormone (PTH) | WB; IHC; ICC; IP. |
| PAA866Ra01 | Polyclonal Antibody to Parathyroid Hormone (PTH) | WB; IHC; ICC; IP. |
| MAA866Ra21 | Monoclonal Antibody to Parathyroid Hormone (PTH) | WB; IHC; ICC; IP. |
| CEA866Ra | ELISA Kit for Parathyroid Hormone (PTH) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| CCA866Ra | CLIA Kit for Parathyroid Hormone (PTH) | Chemiluminescent immunoassay for Antigen Detection. |
| LMA866Ra | Multiplex Assay Kit for Parathyroid Hormone (PTH) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |
| KSA866Ra11 | ELISA Kit DIY Materials for Parathyroid Hormone (PTH) | Main materials for "Do It (ELISA Kit) Yourself". |