Packages (Simulation)

Reagent Preparation
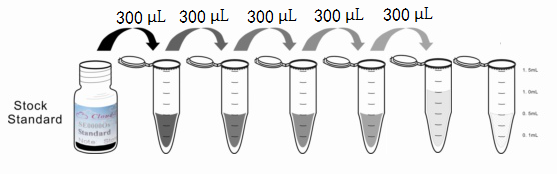
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay)
GOB4; HAG2; XAG2; AG2; PDIA17; HPC8; Anterior Gradient Protein 2; Protein Disulfide Isomerase Family A,Member 17; Secreted cement gland protein XAG-2 homolog
(Note: Up to 8-plex in one testing reaction)
- Product No.LMC285Hu
- Organism SpeciesHomo sapiens (Human) Same name, Different species.
- Sample TypeSerum, plasma, tissue homogenates, cell lysates, cell culture supernates and other biological fluids
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
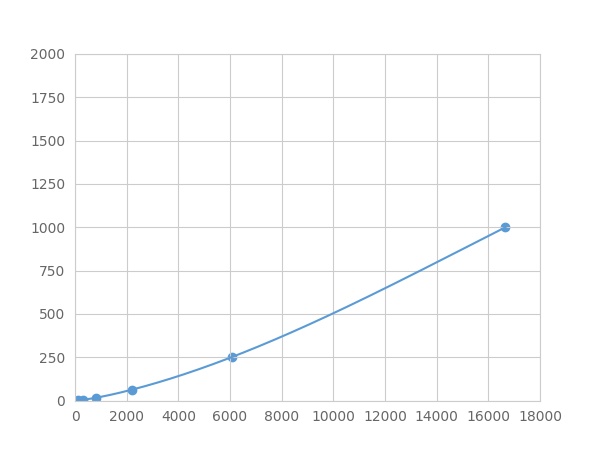
- Detection Range0.98-1000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.327 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 437
US$ 454
US$ 479
US$ 512
US$ 546
US$ 596
US$ 672
US$ 840
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 88-96 | 92 |
| EDTA plasma(n=5) | 79-91 | 84 |
| heparin plasma(n=5) | 91-102 | 99 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 80-95% | 88-95% | 89-96% | 78-97% |
| EDTA plasma(n=5) | 84-98% | 78-95% | 83-101% | 78-94% |
| heparin plasma(n=5) | 88-95% | 87-96% | 90-103% | 79-88% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:AGR2) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Cancer Biomarkers | Serum AGR2 as an early diagnostic and postoperative prognostic biomarker of human lung adenocarcinoma MetaPress: n7137r6271u54118 |
| Molecular & Cellular Proteomics | Integrated Proteomic Profiling of Cell Line Conditioned Media and Pancreatic Juice for the Identification of Pancreatic Cancer Biomarkers PubMed: PMC3205865 |
| Prostate. | Anterior gradient 2 (AGR2): blood-based biomarker elevated in metastatic prostate cancer associated with the neuroendocrine phenotype Pubmed: 22911164 |
| BMC Cancer. | Validation of four candidate pancreatic cancer serological biomarkers that improve the performance of CA19.9 Pubmed: 24007603 |
| Clinical Cancer Research | Validation of Biomarkers That Complement CA19.9 in Detecting Early Pancreatic Cancer Pubmed:25239611 |
| PLoS One | AGR3 in Breast Cancer: Prognostic Impact and Suitable Serum-Based Biomarker for Early Cancer Detection PubMed: 25875093 |
| Clinical Neurology and Neurosurgery | Serum AGR2 as a useful biomarker for pituitary adenomas S0303846717300045 |
| Oncotarget | Identification, characterization and application of a new peptide against anterior gradient homolog 2 (AGR2) Pubmed:29937991 |
| Int J Oncol | Anterior gradient protein 2 is a marker of tumor aggressiveness in breast cancer and favors chemotherapy‑induced senescence escape 34913074 |
| International Journal of Cancer | Proteome profiling of enzalutamide‐resistant cell lines and serum analysis identified ALCAM as marker of resistance in castration‐resistant prostate cancer Pubmed:35689436 |
| Catalog No. | Related products for research use of Homo sapiens (Human) Organism species | Applications (RESEARCH USE ONLY!) |
| RPC285Hu01 | Recombinant Anterior Gradient 2 (AGR2) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAC285Hu02 | Polyclonal Antibody to Anterior Gradient 2 (AGR2) | WB; IHC; ICC; IP. |
| PAC285Hu01 | Polyclonal Antibody to Anterior Gradient 2 (AGR2) | WB; IHC; ICC; IP. |
| LAC285Hu71 | Biotin-Linked Polyclonal Antibody to Anterior Gradient 2 (AGR2) | WB; IHC; ICC. |
| MAC285Hu21 | Monoclonal Antibody to Anterior Gradient 2 (AGR2) | WB; IHC; ICC; IP. |
| LAC285Hu72 | Biotin-Linked Monoclonal Antibody to Anterior Gradient 2 (AGR2) | WB; IHC; ICC. |
| SEC285Hu | ELISA Kit for Anterior Gradient 2 (AGR2) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| SCC285Hu | CLIA Kit for Anterior Gradient 2 (AGR2) | Chemiluminescent immunoassay for Antigen Detection. |
| LMC285Hu | Multiplex Assay Kit for Anterior Gradient 2 (AGR2) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |