Packages (Simulation)

Reagent Preparation
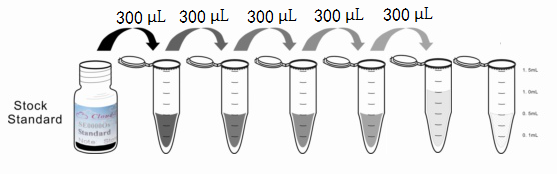
Image (I)
Image (II)
Certificate


Multiplex Assay Kit for Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay)
OSF-2; PDLPOSTN; PN; Osteoblast Specific Factor 2
(Note: Up to 8-plex in one testing reaction)
- Product No.LMH339Ra
- Organism SpeciesRattus norvegicus (Rat) Same name, Different species.
- Sample TypeSerum, plasma, tissue homogenates and other biological fluids.
- Test MethodDouble-antibody Sandwich
- Assay Length3.5h
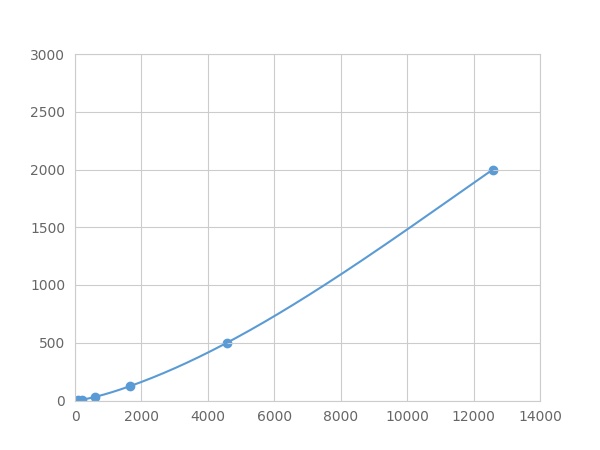
- Detection Range1.95-2000pg/mL
- SensitivityThe minimum detectable dose of this kit is typically less than 0.65 pg/mL.
- DownloadInstruction Manual
- UOM 8Plex 7Plex 6Plex 5Plex 4Plex 3Plex 2Plex1Plex
- FOB
US$ 474
US$ 492
US$ 520
US$ 556
US$ 593
US$ 648
US$ 730
US$ 912
Add to Price Calculator
Result
For more details, please contact local distributors!
Specificity
This assay has high sensitivity and excellent specificity for detection of Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay).
No significant cross-reactivity or interference between Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay) and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay) and the recovery rates were calculated by comparing the measured value to the expected amount of Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay) in samples.
| Matrix | Recovery range (%) | Average(%) |
| serum(n=5) | 80-95 | 85 |
| EDTA plasma(n=5) | 79-97 | 90 |
| heparin plasma(n=5) | 93-101 | 97 |
| sodium citrate plasma(n=5) | 83-97 | 88 |
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, middle and high level Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, middle and high level Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay) were tested on 3 different plates, 8 replicates in each plate.
CV(%) = SD/meanX100
Intra-Assay: CV<10%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay) and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Sample | 1:2 | 1:4 | 1:8 | 1:16 |
| serum(n=5) | 83-97% | 87-94% | 82-103% | 83-101% |
| EDTA plasma(n=5) | 93-102% | 89-102% | 86-95% | 78-101% |
| heparin plasma(n=5) | 93-101% | 97-105% | 96-103% | 85-101% |
| sodium citrate plasma(n=5) | 94-102% | 80-94% | 84-101% | 89-96% |
Stability
The stability of kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
To minimize extra influence on the performance, operation procedures and lab conditions, especially room temperature, air humidity, incubator temperature should be strictly controlled. It is also strongly suggested that the whole assay is performed by the same operator from the beginning to the end.
Reagents and materials provided
| Reagents | Quantity | Reagents | Quantity |
| 96-well plate | 1 | Plate sealer for 96 wells | 4 |
| Pre-Mixed Standard | 2 | Standard Diluent | 1×20mL |
| Pre-Mixed Magnetic beads (22#:POSTN) | 1 | Analysis buffer | 1×20mL |
| Pre-Mixed Detection Reagent A | 1×120μL | Assay Diluent A | 1×12mL |
| Detection Reagent B (PE-SA) | 1×120μL | Assay Diluent B | 1×12mL |
| Sheath Fluid | 1×10mL | Wash Buffer (30 × concentrate) | 1×20mL |
| Instruction manual | 1 |
Assay procedure summary
1. Preparation of standards, reagents and samples before the experiment;
2. Add 100μL standard or sample to each well,
add 10μL magnetic beads, and incubate 90min at 37°C on shaker;
3. Remove liquid on magnetic frame, add 100μL prepared Detection Reagent A. Incubate 60min at 37°C on shaker;
4. Wash plate on magnetic frame for three times;
5. Add 100μL prepared Detection Reagent B, and incubate 30 min at 37°C on shaker;
6. Wash plate on magnetic frame for three times;
7. Add 100μL sheath solution, swirl for 2 minutes, read on the machine.
GIVEAWAYS
INCREMENT SERVICES
| Magazine | Citations |
| Blood | Circulating Periostin Is Elevated In Patients With Hemoglobinopathies and Correlates With Bone Mineral Density In Double Heterozygous Sickle-Cell/Beta-Thalassemia Patients; A Novel Marker Of Bone Strength? Bloodjournal: Source |
| The Journal of Clinical Endocrinology & Metabolism | Serum periostin is associated with fracture risk in postmenopausal women: a 7 years prospective analysis of the OFELY study Pubmed: 24628551 |
| J Clin Endocrinol Metab. | Serum Periostin Is Associated With Fracture Risk in Postmenopausal Women: A 7-Year Prospective Analysis of the OFELY Study Pubmed:24628551 |
| Rheumatology (Oxford). | Circulating periostin levels in patients with AS: association with clinical and radiographic variables, inflammatory markers and molecules involved in bone formation Pubmed:25349442 |
| Journal of Periodontal Research | Assessment of periostin levels in serum and gingival crevicular fluid of patients with periodontal disease Pubmed:25529858 |
| 新医学 | 血清内脂素水平与多囊卵巢综合征的相关性研究 Xinyixue:Source |
| Osteoarthritis | Serum periostin is associated with prevalent knee osteoarthritis and disease incidence/progression in women: the OFELY study PubMed: 26072384 |
| Endocrine | Serum periostin is a potential biomarker for non-alcoholic fatty liver disease: a case– control study PubMed: 26362060 |
| Blood Cancer Journal | High levels of periostin correlate with increased fracture rate, diffuse MRI pattern, abnormal bone remodeling and advanced disease stage in patients with newly … pubmed:27716740 |
| Metabolism. | Low periostin levels in adult patients with Langerhans cell histiocytosis are independently associated with the disease activity. pubmed:28521873 |
| Osteoporosis International | Circulating periostin levels increase in association with bone density loss and healing progression during the early phase of hip fracture in Chinese older women pubmed:28382553 |
| Molecular Diagnosis & Therapy | The Utility of Biomarkers in Osteoporosis Management pubmed:28271451 |
| Journal of Bone and Mineral Research | Cortical and trabecular bone microstructure did not recover at weight-bearing skeletal sites and progressively deteriorated at non-weight-bearing sites during the year following International Space Station missions doi:10.1002 |
| Clinical Cases in Mineral and Bone Metabolism | Periostin and sclerostin levels in juvenile Paget's disease pubmed:29263750 |
| Calcified Tissue International | Development of a New Immunoassay for Human Cathepsin K-Generated Periostin Fragments as a Serum Biomarker for Cortical Bone pubmed:28725907 |
| Wiley | Characterization of a sandwich ELISA for the quantification of all human periostin isoforms pubmed:28493527 |
| International Osteoporosis Foundation and National Osteoporosis Foundation | Interaction between LRP5 and periostin gene polymorphisms on serum periostin levels and cortical bone microstructure pubmed:29038835 |
| Bone | Effect of age and gender on serum periostin: Relationship to cortical measures, bone turnover and hormones pubmed:28323143 |
| Clinica Chimica Acta | Association between serum periostin concentrations and outcome after acute spontaneous intracerebral hemorrhage pubmed:28882488 |
| Clinica Chimica Acta | Serum periostin concentrations and outcomes after severe traumatic brain injury pubmed:28668564 |
| Tumor Biology | Predictive and prognostic value of serum periostin in advanced non–small cell lung cancer patients receiving chemotherapy pubmed:28459197 |
| Journal of Bone and Mineral Research | Serum Levels of a Cathepsin-K Generated Periostin Fragment Predict Incident Low-Trauma Fractures in Postmenopausal Women Independently of BMD and FRAX pubmed:28766739 |
| ОСОБЕННОСТИ СОСТОЯНИЯ ЗДОРОВЬЯ НОВОРОЖДЕННЫХ, РОЖДЕННЫХ ОТ МАТЕРЕЙ С БРОНХИАЛЬНОЙ АСТМОЙ 2017; 62:(4) | |
| Endocrine | Circulating periostin in patients with nonalcoholic fatty liver disease. pubmed:27738886 |
| Molecular Medicine Reports | Periostin cross‑reacts with the renin‑angiotensin system during liver fibrosis development pubmed:28849131 |
| The Journal of Biological Chemistry | Toll-like receptor 2 deficiency hyperactivates the FoxO1 transcription factor and induces aging-associated cardiac dysfunction in mice Pubmed:29929978 |
| Calcified Tissue International | The C-Terminal Intact Forms of Periostin (iPTN) Are Surrogate Markers for Osteolytic Lesions in Experimental Breast Cancer Bone Metastasis Pubmed:29916127 |
| Journal of Endocrinological Investigation | Increased serum periostin concentrations are associated with the presence of diabetic retinopathy in patients with type 2 diabetes mellitus Pubmed:29349642 |
| Wiener klinische Wochenschrift | Rheumatoid arthritis in remission |
| Clinical and Experimental Medicine | Myostatin and other musculoskeletal markers in lung transplant recipients Pubmed: 30317402 |
| Experimental and Clinical Endocrinology & Diabetes | Polycystic Ovary Syndrome is Associated with Elevated Periostin Levels Pubmed: 30372763 |
| ИСПОЛЬЗОВАНИЕ СЫВОРОТОЧНОГО ПЕРИОСТИНА В КАЧЕСТВЕ МАРКЕРА ОБОСТРЕНИЙ АСТМЫ У ДЕТЕЙ | |
| Bone | Tartrate-resistant acid phosphatase 5b, but not periostin, is useful for assessing Paget's disease of bone Pubmed: 31051316 |
| Journal of Clinical Laboratory Analysis | Identification and diagnostic value of pleural fluid periostin and serum periostin of malignant pleural effusions in patients with non–small‐cell lung cancer Pubmed: 31268191 |
| Bone | Serum periostin is associated with cancer mortality but not cancer risk in older home-dwelling men: A 8-year prospective analysis of the STRAMBO study Pubmed: 31812700 |
| FASEB J | Fibronectin rescues aberrant phenotype of endothelial cells lacking either CCM1, CCM2 or CCM3 Pubmed: 32515053 |
| Journal of Reproductive Immunology | Serum and placental periostin levels in women with early pregnancy loss Pubmed: 32460058 |
| Annals of Medical Research | Evaluation of periostin levels in gingival crevicular fluid and peri-implant sulcus fluid in patients with periodontal and peri-implanter disease: A cross-sectional … |
| Myostatin and Markers of Bone Metabolism in Dermatomyositis | |
| Am J Physiol Heart Circ Physiol | Periostin promotes arterial calcification through PPAR¦Ã-related glucose metabolism reprogramming 33834866 |
| International Journal of Chronic Obstructive Pulmonary Disease | High Blood Eosinophil and YKL-40 Levels, as Well as Low CXCL9 Levels, are Associated with Increased Readmission in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease 33814903 |
| J Bone Oncol | Serum total periostin is an independent marker of overall survival in bone metastases of lung adenocarcinoma 34150488 |
| Rambam Maimonides Med J | The Relationship between Serum Periostin Levels and Neutrophil¨CLymphocyte Ratio with Lesion Localization and Acute Prognosis in Acute Ischemic Stroke 34137680 |
| Rambam Maimonides Medical Journal | First Admission Neutrophil–Lymphocyte Ratio May Indicate Acute Prognosis of Ischemic Stroke 34137680 |
| Catalog No. | Related products for research use of Rattus norvegicus (Rat) Organism species | Applications (RESEARCH USE ONLY!) |
| RPH339Ra01 | Recombinant Periostin (POSTN) | Positive Control; Immunogen; SDS-PAGE; WB. |
| RPH339Ra02 | Recombinant Periostin (POSTN) | Positive Control; Immunogen; SDS-PAGE; WB. |
| PAH339Ra01 | Polyclonal Antibody to Periostin (POSTN) | WB; IHC; ICC; IP. |
| PAH339Ra02 | Polyclonal Antibody to Periostin (POSTN) | WB; IHC; ICC; IP. |
| LAH339Ra71 | Biotin-Linked Polyclonal Antibody to Periostin (POSTN) | WB; IHC; ICC. |
| SEH339Ra | ELISA Kit for Periostin (POSTN) | Enzyme-linked immunosorbent assay for Antigen Detection. |
| LMH339Ra | Multiplex Assay Kit for Periostin (POSTN) ,etc. by FLIA (Flow Luminescence Immunoassay) | FLIA Kit for Antigen Detection. |