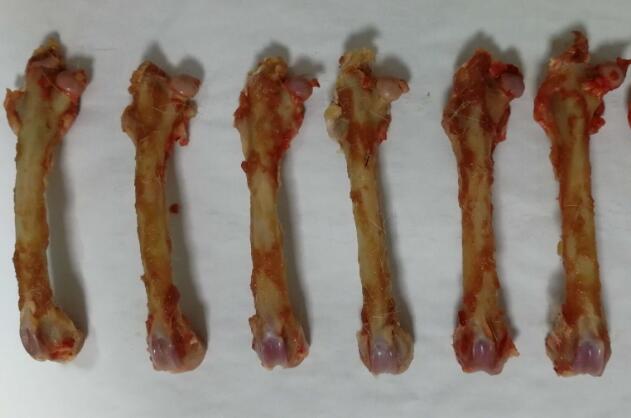
Rabbit Model for Femoral Defect (FD)
Femoral Bone Defect
- Product No.DSI840Rb01
- Organism SpeciesOryctolagus cuniculus (Rabbit) Same name, Different species.
- Prototype SpeciesHuman
- SourceFemoral defect caused by surgery
- Model Animal StrainsJapanese big white rabbit, male, 4 months, 2~3kg
- Modeling GroupingModel group: femoral defect modeling; B positive control group: femoral defect modeling, using autogenous bone transplantation; test group: femoral defect modeling, biomaterial transplantation;
- Modeling Period16w
- Modeling Method1. femoral defect modeling
Surgical procedures: after successful anesthesia, the rabbit hind leg knee joint was slightly flexed, skin was tightened, hair was removed, and 2.5% iodine was disinfected. Sterilize the surgical area with tincture of iodine and ethanol, and spread sterile towels. Avoiding the subcutaneous blood vessels, incise the skin, subcutaneous tissue and fascia obliquely. The incision is 2.0cm to expose the external femoral epicondyle, peel off the attached tendons, and expose the femoral shaft and femoral condyle metaphyseal line. The hand-held dental fracture drill with a diameter of 6mm drilled into a depth of about 1cm to remove bone and periosteum at the defect.
2. Take autologous bone as graft material for positive control
Procedures of autologous bone tissue sampling:At the center of the fifth lumbar spinous process, a dorsal midline incision of about 5.0cm was made. The skin and subcutaneous incision was made to expose the lumbar back fascia. First, the fascia and aponeurosis attached were cut from the inside to the outside along the bilateral iliac crest line. After careful separation of the iliac crest, the internal and external muscles of the iliac bone were separated from the inside to the outside periosteum. The size of each iliac bone wing was about 1.5cm × 2.0cm with a bone knife, and the incision was sutured intermittently. The ilium bone was made into 1mm×1 mm×15 mm single-sided cortical bone strip along the long axis. Autologous bone was collected from animals, and bone defect model was made 2 weeks after surgery. - ApplicationsDisease Model
- Downloadn/a
- UOM Each case
- FOB
US$ 400
For more details, please contact local distributors!
Model Evaluation
The detection points were divided into 4, 8 and 16 weeks after surgery
1. Imaging detection
DR digital X radiographs were performed 1 day before sampling (4, 8 and 16 weeks after surgery). According to the general healing and cortical connection scoring standards, the double-blind principle was used to evaluate the fracture healing in each group and perform imaging scores.
At the same time, the animal’s femoral tissue was detected by micro-CT at 16 weeks after the surgery to analyze the bone density and trabecular bone parameters to observe the repair effect.
2. Cadaver observation
Two animals were sacrificed at 4, 8 and 16 weeks after surgery, observing the degradation of the material and adhesion of surrounding tissues. The cadaver evaluation after sampling shall include the following contents:
a. Appearance description of the defect;
b. Appearance of bone around defect (e.g. presence of callus);
c. Appearance of surrounding bone (e.g. presence of osteophytes);
d.Description of the color and quantity of tissue at the repaired site (including surface appearance and structure), degree of implant integration.
Pathological Results
MicrocT was used to detect femur, analyze BMD, Bone Coverage, trabecular Bone and other information.
The tissue of the bone defect area was cut as the specimen, fixed with 10% formalin or 4% formaldehyde at room temperature for 3 days, and the specimen was analyzed by HE staining after dehydration, embedding and hard tissue sectioning.Histological score was performed.
Histological observation: Osteoblasts, bone trabeculae, blood vessel formation and collagen fiber formation were observed by HE staining and masson, and the histological differences among each experimental group were compared.
Cytokines Level
The new tissue samples were taken from the bone defect area and crushed under liquid nitrogen to extract total protein.
Western blotting was used to detect the expression of alkaline phosphatase (ALP), Runx2 and Osteoclacin.
Statistical Analysis
SPSS software is used for statistical analysis, measurement data to mean ± standard deviation (x ±s), using t test and single factor analysis of variance for group comparison, P<0.05 indicates there was a significant difference, P<0.01 indicates there are very significant differences.
GIVEAWAYS
INCREMENT SERVICES
-
 Tissue/Sections Customized Service
Tissue/Sections Customized Service
-
 Serums Customized Service
Serums Customized Service
-
 Immunohistochemistry (IHC) Experiment Service
Immunohistochemistry (IHC) Experiment Service
-
 Small Animal In Vivo Imaging Experiment Service
Small Animal In Vivo Imaging Experiment Service
-
 Small Animal Micro CT Imaging Experiment Service
Small Animal Micro CT Imaging Experiment Service
-
 Small Animal MRI Imaging Experiment Service
Small Animal MRI Imaging Experiment Service
-
 Small Animal Ultrasound Imaging Experiment Service
Small Animal Ultrasound Imaging Experiment Service
-
 Transmission Electron Microscopy (TEM) Experiment Service
Transmission Electron Microscopy (TEM) Experiment Service
-
 Scanning Electron Microscope (SEM) Experiment Service
Scanning Electron Microscope (SEM) Experiment Service
-
 Learning and Memory Behavioral Experiment Service
Learning and Memory Behavioral Experiment Service
-
 Anxiety and Depression Behavioral Experiment Service
Anxiety and Depression Behavioral Experiment Service
-
 Drug Addiction Behavioral Experiment Service
Drug Addiction Behavioral Experiment Service
-
 Pain Behavioral Experiment Service
Pain Behavioral Experiment Service
-
 Neuropsychiatric Disorder Behavioral Experiment Service
Neuropsychiatric Disorder Behavioral Experiment Service
-
 Fatigue Behavioral Experiment Service
Fatigue Behavioral Experiment Service
-
 Nitric Oxide Assay Kit (A012)
Nitric Oxide Assay Kit (A012)
-
 Nitric Oxide Assay Kit (A013-2)
Nitric Oxide Assay Kit (A013-2)
-
 Total Anti-Oxidative Capability Assay Kit(A015-2)
Total Anti-Oxidative Capability Assay Kit(A015-2)
-
 Total Anti-Oxidative Capability Assay Kit (A015-1)
Total Anti-Oxidative Capability Assay Kit (A015-1)
-
 Superoxide Dismutase Assay Kit
Superoxide Dismutase Assay Kit
-
 Fructose Assay Kit (A085)
Fructose Assay Kit (A085)
-
 Citric Acid Assay Kit (A128 )
Citric Acid Assay Kit (A128 )
-
 Catalase Assay Kit
Catalase Assay Kit
-
 Malondialdehyde Assay Kit
Malondialdehyde Assay Kit
-
 Glutathione S-Transferase Assay Kit
Glutathione S-Transferase Assay Kit
-
 Microscale Reduced Glutathione assay kit
Microscale Reduced Glutathione assay kit
-
 Glutathione Reductase Activity Coefficient Assay Kit
Glutathione Reductase Activity Coefficient Assay Kit
-
 Angiotensin Converting Enzyme Kit
Angiotensin Converting Enzyme Kit
-
 Glutathione Peroxidase (GSH-PX) Assay Kit
Glutathione Peroxidase (GSH-PX) Assay Kit
-
 Cloud-Clone Multiplex assay kits
Cloud-Clone Multiplex assay kits
| Catalog No. | Related products for research use of Oryctolagus cuniculus (Rabbit) Organism species | Applications (RESEARCH USE ONLY!) |
| DSI840Rb01 | Rabbit Model for Femoral Defect (FD) | Disease Model |
| TSI840Rb69 | Rabbit Femur Tissue of Femoral Defect (FD) | Paraffin slides for pathologic research: IHC,IF and HE,Masson and other stainings |